GRADE 10 BIOTECHNOLOGY CURRICULUM
advertisement

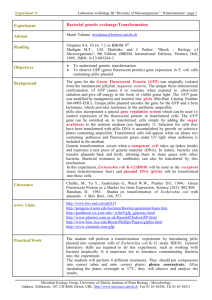
Grade 10 Biotechnology Cycles 1-20 Cycle 1 2 3 4 Cycle Overview Lab safety and assessment MSDS sheets Fundamentals of Botany. Plant histology: Wet mount slide preparation and observation, Flower anatomy, Dicots vs. Monocots, Plant embryology, meristems. Plant cloning: rooting hormone studies, germination set up, use of tissue culture hood Microscopy: proper use of microscope, Compare and contrast stereo microscope vs. light microscope Botany continued: identify plants as dicots or monocots, prepare slides from onion root tips and observe mitotic domains. Enzymology: tissue specific peroxidase assay, peroxidase isolation, assay and PAGE analysis. Plant cloning: using aseptic technique, transfer meristem from seedling to callus media, observe and document rooting hormone results. Plant cloning will continue throughout semester. Students will document results and transfer tissues to specialized media as necessary Molecular genetic techniques: Prepare competent E.coli JM109 cells for plasmid transformation using the calcium chloride method. Interpret a plasmid map. Understand the use of selectable markers. Plasmid transformation experiment and induction of Green Fluorescent Protein (GFP). Set up a liquid overnight culture from a single JM109 pGLO colony. Freeze cell pellet for DNA preparation in cycle 5. Molecular genetics continued: Prepare competent cells and transform with plasmid pBR322 Research and present a specific plasmid and its uses. Use of nanoparticles in Biotechnology. Visit the Harvard Univ. Center for Nanosystems. 5 Molecular genetics: prepare plasmid DNA using the alkali lysis method. Streak for single colony isolation and label correctly: pBR322 and PGLO containing JMIO9 stocks. Perform a restriction digest to linearize plasmid DNA. Compare pBR322 and PGLO DNA using gel electrophoresis. Observe migration differences between linearized, nicked circle and supercoiled DNA samples. 6 DNA restriction maps: pBR322 and pGLO. Diagnostic restriction enzyme digests of cycle 5 plasmid preps. Create and use a restriction map to identify plasmid DNA. 7 8 Introduction to pH Prepare for GFP isolation from pGLO strain: Assay induction conditions. Thanksgiving week theme: genetics of taste and assay. Development of lactase persistence in humans and lactase assay development. pH solutions of acidic and basic amino acids Grow and induce GFP expression from JM109 pGLO. Prepare bacteria cell lysate from induced cells. Create and compare bacteria growth curves of induced and uninduced cells. Relate number of colonies to cell density and optical density. Perform a plasmid loss assay for cells grown with and without antibiotic. 9/10 11 12/13 14 15 16 17 18 Preteach for next cycle: pH and protein charge. Column chromatography Enrich for GFP using a hydrophobic interaction column/ dialysis of eluate. Separate proteins using ion exchange chromatography. Explain principles of chromatography showing an understanding of hydrophobicity, pI, pH, ionic interactions and other necessary concepts. Semester 1 Final shop exam. Chloroplast Isolation: density gradient centrifugation, sucrose step gradient, use of the hemacytometer. Chromatography of chlorophyll Development of functional assay using pH indicator Extrapolating organelle density from hemacytometer counts. Review for next week: PCR theory. Lagomorph species identification using DNA isolation and analysis: DNA isolation, use of mitochondrial DNA for species identification, enzyme site polymorphisms, gel analysis of DNA fragment polymorphisms, PCR, optimal enzyme conditions. Identification of bacteria species: use of differential vs. selective media, types of bacteria fermentation and products, pH indicators, metabolic differences between gram negative and gram positive bacteria and relation to cell wall structure, salt tolerance and metabolism. Vibrio harveyi and quorum sensing. Assay development for antibiotics Elisa assays and western blots Introduction to Immunology Antigen Antibody interactions. Comparisons of secondary antibodies used for detection Use and comparison of different transfer apparatuses. Term 3: written final Shop assessment Identification of foods produced from genetically modified plants. Aromatic amino acid synthesis and Round up herbicide Vectors for plant transformation Process of creating transgenic plants PCR review Environmental/Ethical considerations. Amylase assay and purification Differential centrifugation Purification protocol development Assay development Enzyme kinetics Optimal enzyme conditions Crime scene investigation techniques Forensic application of the PCR Understanding genetic polymorphisms and CODIS Population genetics Literature circle: Supreme court ruling on DNA testing at crime scenes. 19 20 Make-up labs from snow days/ field trip etc. Competency Assessments: micropipets, balance, autoclave, still, incubators, spectrophotometer, excel, pH meter, agarose gel preparation, solution prep,microbiology techniques. Lab inventory and ordering Lab clean up Written exam: final Shop assessment. Make Soap