13APR1WB_OEL Webinar Glossary 4-24
advertisement

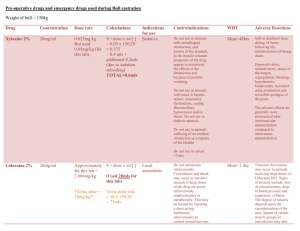
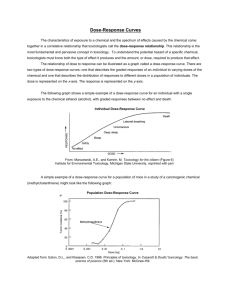
GLOSSARY Term BMD CSAF DNEL Dose-Response Curve EDM False Threshold DoseResponse Harmonization of Risk Methods Hazard Quotient HEMP Hormesis HRA IURF Linearized DoseResponse LOAEL Definition Benchmark Dose. A dose or concentration that produces a predetermined change in response rate of an adverse effect (called the benchmark response or BMR) compared to background. The lower bound statistical estimate of dose associated with the BMR is the BMDL. The BMDL is often used as a point of departure for deriving an OEL. Chemical Specific Adjustment Factor. A methodology to use chemical-specific data on toxicokinetics (the disposition of a chemical in the body) and toxicodynamics (the impact of the chemical dose at the level of the target in the body) to inform human variability in susceptibility and interspecies differences in response to chemicals. The approach is used to replace default uncertainty factors for intraspecies and interspecies variability for deriving exposure guide values such as OELs. Derived No Effect Level. The level of exposure above which humans should not be exposed. The DNEL is used as part of the risk characterization as the health effect benchmark in the European Community regulation on chemical registration or REACH (Registration, Evaluation, Authorization and Restriction of Chemicals). The estimated relationship between the changes in the level of toxic response over a series of exposures or doses. Dose-response curves can cover different portions of the relationship starting at zero dose and extending through higher doses that might induce frank or clinically apparent toxicity. Exposure Data Management A dose-response pattern for which risk at very low dose increase rapidly from zero exposure and then decreases in a non-monotonic manner until the response is close to zero and then it proceeds upward monotonically to high toxicity at high dose. Increasing understanding and developing basic principles and guidance on specific chemical risk assessment issues. Harmonization enables efficient use of resources and consistency among assessments. For additional information see the International Programme on Chemical Safety Harmonization Project site). A ratio of the measured or estimated exposure and the occupational exposure limit or other exposure guide value. As values reach and exceed unity concern for potential health risk increases. Hazard and Effects Management Process A decreasing dose response at doses up from zero in which there is actually a stimulatory response from the exposure such that there is a beneficial effect on health. Example: some studies have indicated that one or two drinks of alcohol seem to be somewhat protective of heart disease. Health Risk Assessment Inhalation Unit Risk Factor. The upper- bound excess lifetime cancer risk estimated to result from continuous exposure to an agent at a concentration of 1 ug/m 3 in air. This term is often used for cancer risk assessments for the general populations exposed by the inhalation route. A method of extrapolating to estimate toxic responses outside the range of the data in the low dose region that is often applied for biological effects that might be induced without a true threshold. That is, some adverse effect is predicted at the lowest exposure and this response increases linearly with dose such that twice any exposure is equal to twice the predicted risk. This is a common technique for cancer risk assessments. Lowest Observed Adverse Effect Level. The lowest tested dose or concentration of a substance that has been reported to cause harmful (adverse) health effects in people or animals. Monotonic A dose response which is continuously increasing or decreasing with increasing dose. NOAEL No Observed Adverse Effect Level. A tested dose or concentration of a substance that has not been reported to cause harmful (adverse) health effects in people or animals. OEL Occupational exposure limit – an exposure during employment activity that is considered to not present an unreasonable risk for a working lifetime as determined by those who set it. It is usually applicable as a ceiling limit or a time-weighted average for 15 minutes (short-term) or 8 hours per day (full-shift), 5 days a week, 50 weeks/year for a working lifetime. OEL-D The level of quantitative risk estimated at any current OEL provided as part of the OEL documentation. POD Point of Departure. The point on the dose-response curve from which dose extrapolation is initiated. This point can be the lower bound on dose for an estimated incidence or a change in response level from a dose-response model (BMDL), or a NOAEL or LOAEL for an observed effect selected from a dose evaluated in a health effects or toxicology study. RBOEL Risk Based Occupational Exposure Limits – limits set based on a quantitative estimate of residual risk present for exposure at the exposure limit. Often set at 1 in 1000. RfC Reference Concentration. An estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure for a chronic duration (up to a lifetime) to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL, or benchmark concentration, with uncertainty factors generally applied to reflect limitations of the data used. Generally used in U.S. Environmental Protection Agency (EPA) non-cancer health assessments for exposure by the inhalation route. Threshold DoseResponse A method of dose-response assessment that is often applied for biological effects that have a finite dose or threshold below which there is no expected adverse effect on anyone in the exposed population. This is a common assumption for risk assessments for most non-cancer effects. Uncertainly Factors: Mathematical adjustments applied to the POD when developing IDLH values. The uncertainty factors for IDLH value derivation are determined by considering the study and effect used for the POD, with further modification based on the overall database. Weight-of-Evidence. Extent to which the available biomedical data supports a conclusion, such as whether a substance causes a defined toxic effect, whether the effect observed is relevant to human risk, or whether an effect occurs at a specific exposure level of concern. The WoE concept includes the integration of the totality of all lines of evidence considering their relevance and reliability to support an overall conclusion. Uncertainty Factors WoE AIHA Webinar: The New Era of Global Exposure Limit Setting Processes – Harmonization on an OEL Hierarchy Approach Date presented: April 11, 2013 Presenters: Chris Laszcz-Davis, MS,CIH; Karen Niven, PhD, MSc, RSP, CFIOSH, FFOH; Michael Jayjock, PhD, CIH; Andrew Maier, PhD, CIH, DABT; Renee Kalmes, MSPH, CIH; Susan Ripple, MS, CIH; Donna Heidel, MS, CIH