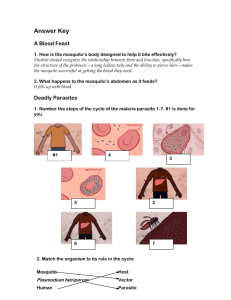
C. oleophila inhibits P. falciparum development in
advertisement

Investigation of the anti-Plasmodium properties of the Yeast Candida oleophila in the Malaria Mosquito, Anopheles gambiae by George Edward “Ned” Barringer III A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science Baltimore, Maryland April,2014 Abstract Plasmodium falciparum is a causative agent of malaria and a significant global health burden. The primary vector of P. falciparum in sub-Saharan Africa is the Anopheles gambiae species complex. Plasmodium species infect approximately 500 million people and cause nearly 1 million deaths annually. Current control efforts are hampered by drug resistant P.falciparum parasites and insecticide resistant mosquitoes. In the continuing effort to control malaria infection, microbes native to the midgut are being studied for species that can inhibit P. falciparum. While the bacteria of the mosquito microbiome have been well studied, few studies have explored fungi and yeast in the mosquito microbiome. Presented here is research on a Candida oleophila yeast isolated from the An. gambiae midgut that shows significant inhibitory effects on the P. falciparum parasite in vivo. We have assessed the inhibition of P. falciparum development in vivo and in vitro, as well as the impact of the presence of this yeast on the midgut bacterial flora and vector survival. This yeast isolate inhibits P. falciparum in a pre-oocyst stage through a secreted factor; numbers of oocysts are significantly reduced in those An. gambiae harboring yeast or supernatant filtrate in their midguts. An. gambiae harboring yeast in their midgut experience expansions in midgut bacteria populations, but no change in cohort survival was observed. Primary Reader: Dr. George Dimopoulos Secondary Reader: Dr. Clive Shiff II Acknowledgements First and foremost, I have to thank Dr. George Dimopoulos, for accepting me into his dynamic and exciting laboratory. His encouragement and guidance were crucial in helping me grow as a research scientist. I also want to thank my thesis reader, Dr. Clive Shiff for excellent suggestions both on my experiments and my thesis even when time was limited. My thanks go to my colleagues in the Dimopoulos laboratory: Sarah Short, Pike, Nathan Dennison, Yang Chen, Raul Saraiva, Tui, Yesseinia Anglero-Rodriguez, Seokyoung Kang, Yuemei Dong, Simone Sandiford, and Alicia Majeau. I especially want to thank Ben Blumberg for expert teaching of mosquito research techniques and many interesting discussions which enriched my study. Additionally, I must thank Godfree Mlambo and Anne Jedlicka, for use of the P. berghei ookinete system and training on Quantitative real time PCR, respectively. Last but not least, I give a special thanks to my family. My parents George E. Barringer, Jr. and Nancy Franzen Barringer have offered continual support through my education, and I would not be where I am today without them. III Table of Contents Abstract………………………………………...……………………………………..…..II Acknowledgements…………….…………………….…………………………………..III Table of Contents ……………………………….……………………………………….IV Table of Figures ...……………………………….………………………………………VI Chapter1: Introduction………...………………………………………………………..1 History………………………………………………………………………………...1 Plasmodium Species and Distribution………………………………………………...1 Plasmodium falciparum Biology……………………………………………………...2 Anopheles gambiae Species Complex ………………………………………………..5 Antimalarial Drugs and Vaccines ………………………………………………….....6 Plasmodium falciparum Control Using Genetically Modified Organisms…………..10 Mosquito Immune System ………………………………………………………….11 Mosquito Microbiome ……………………………………….……………………..13 Chapter 2:Methods……………………………………………………………………. 18 Rearing Anopheles gambiae ………………………………………….……………..18 Yeast Isolates……………………………………………………………………...…18 Sequencing…………………………………………………………………………...18 Mosquito Feeding with Yeast and Supernatant……………………………………...19 Plasmodium Challenge……………………………………………………………....19 Quantification of Midgut Bacteria……………………………………………….…..20 In Vitro P. berghei Ookinete Inhibition Assay……………………………………....20 IV Longevity Assay……………………………………………………………………..21 Data Analysis……………………………………………………………………...…22 Chapter 3:Results…………………………………………….………………………... 23 Isolation and Sequencing of Candida………………………………………………..23 The Presence of C. oleophila in the Mosquito Midgut Inhibits P. falciparum Infection ……………………………………………………………………………………......23 Oocyst Development is Inhibited in the Mosquito Midgut by a C. oleophila -Secreted Factor………………………………………………...………………........................25 C. oleophila Does Not Inhibit Plasmodium berghei Ookinete Development in vitro ………………………………………………………………………………………..27 Presence of C. oleophila Results in an Increased Bacterial Load of the Mosquito Midgut………………………………………………………………..……….……..28 The Presence of C. oleophila in the Midgut Does Not Influence Mosquito Longevity…………………………………………………………………………….29 Chapter 4: Discussion……………………………..…………………..………………..31 References …………………………...………...………………………………………..38 V List of Figures Figure 1.Global Distribution of Clinical Malaria Cases ……………….…………………2 Figure 2. Plasmodium Falciparum Lifecycle ….……………………...………………….4 Figure 3. Fold Change of Plasmodium Parasites at Various Stages in the Mosquito ...…..5 Figure 4. The Range of Potential and Current Malaria Vectors Worldwide ……………..6 Figure 5. The Impact of C. oleophila Presence in the Midgut on P. falciparum Oocyst Development in vivo ……………………………………..…………...….………...…....25 Figure 6. C. oleophila Culture Supernatant Filtrate Inhibits Development of Oocysts in An. gambiae ………………………………………………………….…………..……...26 Figure 7. There is no significant difference in ookinete development as concentration of C. oleophila spores increases ….…………………………………………..…………….27 Figure 8. The Presence of C. oleophila in the An. gambiae Midgut Increases the Bacteria Load of the Midgut ……………..………………………………...……………………..29 Figure 9. There is No Significant Difference Between Control and Yeast Fed Mosquitoes Survival …………………………………………...………………………………….….30 VI Introduction History Malaria is one of the oldest recorded diseases, with medical practitioners from ancient China, Greece, and Roman Empire writing about the disease. The oldest recorded mention of the disease is in an ancient Chinese medical text. The Greek medical practitioner Hippocrates recording the symptoms of the disease in detail: fever, splenomegaly, and anemia [1]. Hippocrates was the first to note the connection between the symptoms of malaria and the proximity to swamps[1]. The Romans, as well, appreciated the relation of malarial symptoms to swamps and their knowledge led to large scale swamp draining that prevented malaria from becoming a massive impediment to Roman citizens [2]. Although the connection to swamps and warm weather was known for millennia, the causative agent of malaria was thought to be miasma from swamps. The term malaria derives “mal aria” or bad air [2]. It was thought that the foul air from marshy areas would cause illness in those that lived nearby. It was only in the late 1800s that Plasmodium falciparum was determined to be an etiological agent of malaria [2]. Plasmodium Species and Distribution Malaria is a disease caused by protozoan parasites of the genus Plasmodium. Plasmodium species infect a wide variety of macroorganisms including rodents, birds, and primates. There are five species of Plasmodium that infect humans. The species vary in range and vector and are P. falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae. Plasmodium knowlesi is a simian parasite, but is also known to infect humans [3]. P. falciparum is the most deadly of these five species, and is 1 currently endemic in South and Central America, Africa, and Asia (Fig 1) [3]. Grey areas represent areas of unstable transmission (Fig 1). The large geographic area which P. falciparum affects and the relative poverty of most of those endemic regions make the elimination of the disease difficult [3]. Figure 1. The global distribution of clinical episodes of Plasmodium falciparum malaria in 2007 [4]. Plasmodium falciparum Biology Plasmodium species are transmitted in a cycle between an avian, reptilian, or mammalian host and a vector mosquito. Vector mosquitoes of Plasmodium are from several different genera; P. falciparum is transmitted by mosquitoes of the genus Anopheles [5]. Humans are infected with P. falciparum sporozoites that are injected into the skin with saliva by a feeding female mosquito. Sporozoites make their way through the skin via capillaries to the circulatory system. The sporozoites rapidly migrate to the liver where they infect liver cells [6]. Inside a liver cell, a sporozoite will begin asexual 2 multiplication of merozoite stage parasites. The cell ruptures following multiplication and the merozoites enter the blood stream and infect red blood cells. When a merozoite invades a red blood cell, it multiplies inside the cell [6], [7]. The blood cell will rupture and release merozoites into the blood stream. The characteristic pathology seen in P. falciparum malaria is a result of blood stage parasites rupturing red blood cells and adhesion of parasitized red blood cells to the vasculature [8]. While many merozoites go on to infect other red blood cells, a number of parasites differentiate into sexual cells called gametocytes. Gametocytes do not cause human pathology, and will remain in the blood stream until taken up by a mosquito or killed by either human immune system or drugs [7]. When a mosquito takes a blood meal from the infected host, gametocytes are taken up into the female mosquito’s midgut. The change in temperature from the mammalian host to the mosquito causes microgametocyte exflagellation [7], [8]. While in the midgut, the now mobile microgametes and the macrogametes fuse on contact. The result is a highly mobile parasite called an ookinete. The ookinete will bind the midgut epithelium and penetrate to the basal side of the midgut. The ookinete develops into an oocyst that remains attached to the basal side of the midgut for several days [7], [8]. During the time the oocyst is attached to the midgut, sporozoites develop inside the oocyst before rupturing the oocyst. The sporozoites then migrate through the mosquito hemocoel to the salivary gland, from which they can infect another human. [7], [8]. The length of time from the initial infection with P. falciparum gametocytes to the time sporozoites migrate to the salivary gland is approximately 14 days [7], [9]. 3 This study focused on the mosquito stages and specifically on the parasites infection of the midgut tissue (fig. 2). Inhibiting the parasite in the mosquito would offer a novel approach to controlling malaria. Approaches to inhibiting the parasite in the mosquito will be described later in this introduction. Figure 2. The complete lifecycle of Plasmodium falciparum. The mosquito stages are represented on the left hand side of the diagram, while the human stages are shown on the right [8]. The numbers of live P. falciparum vary as the parasite migrates through the mosquito. As the gametocytes enter the food bolus in the midgut and mate, about there is a significant reduction in the number of viable parasites (fig 3). In vitro culture studies indicate an over 300-fold decrease in parasites from the gametocyte stage to the ookinete stage [10]. As these ookinetes traverse the midgut, many will be eliminated. On average, only 1 to 5 will survive and form oocysts in a natural infection [7], [10]. However, 4 throusands of sporozoites will develop in each oocyst and become released in the hemocoel when those few oocysts burst [10]. Gametocytes ≈300x reduction ookinetes ≈70x Reduction oocysts Undetermined Proliferation Sporozoites Figure 3. Fold Change of Plasmodium Parasites at Various Stages in the Mosquito [10]. Anopheles gambiae Species Complex The Anopheles gambiae species complex is a group of closely related vectors of P. falciparum in found in sub-Saharan Africa. An. gambiae and closely related species predominate in the humid regions of sub-Saharan Africa, breeding in a variety of habitats including rice fields and irrigation channels. (Fig 1) [11]. Knowledge of the Anopheles malaria vector geographic prevalence is important for malaria control programs so that limited public health budgets can be directed to save the greatest number of lives [12], 5 [13]. Additionally, with the changes in global climate the geographic range of the An. gambiae complex may change significantly [3], [13]. According to climatological modeling, increased humidity in currently arid locations would allow these potent vectors to extend their geographic range [13]. Figure 4. The range of potential and current malaria vectors worldwide. An. gambiae is found in a large geographic area across the middle of Africa south of the Sahara Desert [15]. Antimalarial Drugs and Plasmodium falciparum Vaccines The number of deaths attributed to malaria is a current area of controversy, as studies by Lozano and Murray contend that previous work based on WHO World Malaria Report numbers underestimates the actual burden of malaria [16],[17]. The WHO 6 Annual Malaria Report indicated that an estimated 135-287 million clinical episodes of malaria and 627,000 deaths worldwide in 2012, with P. falciparum being the most lethal of the Plasmodium species that infect humans [18]. While deaths due to malaria have decreased since 2004 due to improved malaria control, the disease remains a major cause of mortality especially for African children under the age of 5 [18]. Current malaria control comprises three main strategies: environmental management, anti-Plasmodium therapeutic and transmission blocking drugs, and use of insecticides. Environmental management refers to human made changes to the natural and built environment to reduce vector interactions with people [19]. These interventions are potentially effective, but often require large expenditures due to redesigning infrastructure. Drugs have the advantage of treating individuals at the time of illness, and some drugs such as artemisinin can inhibit gametocytes, reducing or preventing transmission [20]. Insecticides and insecticide treated bednets are both highly effective in preventing individuals from coming into contact with infected mosquitoes [21]. Unfortunately, with few exceptions resistance has developed to drugs as well as insecticides [21]. Thus far, the most successful vaccine for P. falciparum has approximately 25% efficacy. Environmental management in the context of malaria refers to modifying the environment to reduce the transmission of the disease. Various methods can be used depending on the circumstances, including the swamp draining done by the Romans, drains to avoid standing water, and keeping canals clear so that the water flows too fast for mosquitoes to lay eggs in it [22]. These modifications have been shown to be effective in reducing malaria incidence, but have distinct disadvantages. The large scale 7 environmental modification is costly and labor intense [19]. High initial expenses may be difficult for some poorer countries to sustain [19]. Perhaps because of cost or because of the successes of drugs including chloroquine and artemisinin, environmental management has not been widely used for reducing transmission since the early 20th century [22]. Chloroquine was used heavily after it was developed in 1934 by Bayer scientists, and was distributed for clinical use in 1947 [23]. This drug was extremely effective in curing P. falciparum malaria, but P. falciparum evolved resistance to the drug by the 1950s [23]. By the early 1990s, all sub-Saharan African countries had reported that there was significant chloroquine resistance in local P. falciparum strains. In Malawi, for example, treatment with chloroquine had a roughly 50% failure rate by 1993 [24]. The high failure rate reduced the use of this drug, and the availability of other drugs reduced usage of chloroquine greatly in most African countries by the mid-2000s [24]. The current WHO recommended therapy for P. falciparum malaria is artemisinin combination therapy (ACT) [25]. Artemisinin is a compound that was isolated from the traditional Chinese medicinal herb, Artemisia annua. This compound is well tolerated and rapidly clears the malaria parasite from infected individuals [20]. It has been determined that artemisinin acts on asexual stages of the P. falciparum parasite, and as a result, it is useful in reducing the chance of clinical complications due to blood stage parasites binding to the vascular epithelium [20]. Not only does arteminsinin reduce symptoms by killing the asexual stages of the parasite, it also kills the gametocyte stage of P. falciparum that is taken up by mosquitoes [20]. This is very important because this drug can decrease the transmission of P. falciparum if there is sufficient distribution of the drug to endemic areas [26]. Alone, artemisinin and its derivatives require a seven day 8 course for adequate treatment, and monotherapy has been discouraged by the WHO due to the potential for development of resistance [27]. ACT combines an artemisinin derivative with a drug such as lumefantrine, mefloquine, or sulfadoxine/pyrimethamine that is slowly eliminated from the body [27]. The ACT treatment requires a three day course, increasing compliance with the drug regimen compared with a seven day regimen [20], [27]. While current treatments are effective in combatting malaria when used properly, the current high rates of P. falciparum transmission and drug resistance to many drugs such as chloroquine indicates that drugs alone will not be sufficient to meet targets of malaria control [24]. Ideally, a vaccine could be developed to protect individuals from P. falciparum malaria. Glaxo-Smith Kline and Walter Reed Army Institute of Research have developed a vaccine known as RTS,S that is a promising tool to protect against malaria. RTS,S contains circumsporozoite proteins from the sporozoite stage combined with Hepatitis B virus surface antigens and a chemical adjuvant [28]. The makers intend the vaccine to induce a strong immune response to the sporozoite stage with the goal of preventing sporozoite liver infection [28]. The Phase III trials of this vaccine showed 25%-30% efficacy in infants in its most recent trial [28]. This vaccine can greatly reduce the infection among children under 5, the age group suffering the greatest malaria mortality [28]. While several classes of insecticide have been used to reduce An .gambiae populations, pyrethroids are the most commonly used insecticides on insecticide treated bednets [29]. Pyrethroids are attractive for malaria control inside homes because this class of insecticide has a very low toxicity to mammals [29]. Since An. gambiae seeks 9 hosts for blood feeding during the night, the bednet reduces the likelihood of a mosquito reaching a host and thereby reduces the transmission of malaria [30]. Coating the bednet with insecticide has the additional benefit of killing those mosquitoes that come into contact with the net. Unfortunately, mosquitoes have developed resistance to pyrethroids in locations where they are heavily or indiscriminately used [31]. This insecticide resistance jeopardizes future malaria control efforts using pyrethroid treated bednets [32], [33]. Plasmodium falciparum Control in the Mosquito using Genetically Modified Organisms Thus far, there has not been a single control strategy that focuses on reducing the burden of Plasmodium in the mosquito host deployed to the field. Within the mosquito, the Plasmodium parasite has several vulnerabilities that can be exploited. One important target for intervention is the bottleneck at the pre-oocyst stage of development where ookinetes are traversing the midgut [7]. Parasite numbers decrease dramatically by the oocyst stage; since there are so few oocysts compared to the other stages, there will be a better chance of blocking infection at the early oocyst stage [7]. Examples of strategies to block Plasmodium infection of the mosquito are: genetic modification of the mosquito itself to express anti-Plasmodium effector molecules; genetic modification of a microbial species that will be introduced to the mosquito, known as paratransgenesis; and introduction of an unmodified microbial species to the midgut that inhibits Plasmodium development. 10 Research groups such as Jacobs- Lorena lab have developed both genetic and paratransgenic tools to inhibit P. falciparum in the mosquito. A promising genetic strategy developed by the Jacob-Lorena lab modifies the mosquito to produce the Salivary and Midgut Peptide 1 (SM1). SM1 produced by the mosquito inhibits P. falciparum by blocking a receptor in the midgut believed to be responsible for ookinete binding, preventing ookinetes from traversing the midgut [34]. Other approaches genetically modify microorganisms that live within the mosquito. These organisms could secrete anti-Plasmodium factors to inhibit the parasite as it makes its way through the mosquito. Two examples being researched by the Jacobs-Lorena lab are the natural peptide Scorpine and the synthetic peptide Shiva-1 [35]. Both molecules show robust killing of the parasite in vivo, and genes for the two peptides could be transformed into microbes of the mosquito midgut to kill the malaria parasite [35]. These approaches are promising, but not ready for field applications. Mosquito Immune System The immune system of An. gambiae is highly effective in reducing P. falciparum numbers, and approaches to control P. falciparum should leverage the mosquito’s immune response to enhance parasite killing. There are two broader types of mosquito immune response; the first is a humoral response that activates production of small peptides and other effector molecules. The second is cell-mediated, and is mediated by the mosquito hemocytes. One well studied humoral response is the production of the complement-like factor TEP-1, which is important in anti-Plasmodium defense [37]. TEP1 is a thioester containing protein that participates in the process of melanizing 11 certain Plasmodium species, including Plasmodium berghei and P. falciparum [36], [37],[38]. An. gambiae strains refractory to Plasmodium have distinct TEP1 alleles compared to susceptible strains, indicating that melanization is important in Plasmodium control and lysis [37]. The cellular response is also important in the control of P. falciparum. There are several types of hemocytes within the hemocoel, with the phagocytosic granulocyte being the most common hemocyte cell type [38]. These granulocytes can phagocytose harmful microorganisms in the circulatory system and destroy them [38]. Not only do hemocytes function to phagocytose and lyse pathogens, they also are believed to be responsible for immune priming in the mosquito [37] [39], [40], [41]. Immune priming is a memory-like mechanism that increases killing of specific pathogens including P. falciparum upon a second challenge. Whether humoral or cellular, immunological control is mediated through four immune signaling pathways. There are four main immune pathways that have been characterized in Drosophila that also exist in Anopheles: Toll, IMD, JAK-STAT and JNK [42]. The Toll pathway directs responses to fungal pathogens in addition to inhibiting gram positive bacteria [43], [44]. JNK signaling has been demonstrated to induce molecules involved in nitration of epithelial surfaces [45]. Nitration modifies that parasite and stimulates lysis of the parasite by complement-like factors [45]. The 7 members of the STAT transcription factor family regulate the production of Nitric Oxide Synthetase (NOS) following STAT activation by a Janus Kinase (JAK) [46]. Gupta and colleagues determined that the JAK-STAT pathway is important in defense against P. falciparum infection by reducing oocyst survival [46]. The IMD pathway is canonically responsible for defense against gram negative bacteria and P. falciparum in the midgut [40], [42]. 12 In the mosquito, the IMD pathway functions to control the microbial load of the mosquito midgut [42]. Components of the IMD pathway sense components of gram negative bacteria and act through an NF-κB like transcription factor to modulate immune responses [42]. When the mosquito takes a bloodmeal, midgut bacteria proliferate and the IMD pathway is activated to control this bacterial population through anti-bacterial effectors [42]. It may be that Plasmodium defense is a byproduct of maintaining the midgut homeostasis [42]. If Plasmodium is present, effectors of the IMD pathway can act against the pre-oocyst stages of P. falciparum [42]. Notable IMD regulated components include LRRD7, FBN9, and the aforementioned TEP1 [37]. RNA silencing assays show that knockdown of these components reduces Plasmodium inhibition in the mosquito [37]. In addition to IMD effectors, Plasmodium infection is also inhibited by the mosquito’s natural microbiome. Mosquito Microbiome Mosquitoes, like other insects, have a diverse microbial flora. There are high concentrations of microbes in the mosquito midgut, with bacteria being majority of the described organisms [47]. Just as the microbiome of higher organisms is located through diverse organ systems and tissues, the insect microbiome is located across multiple compartments [47]. Wolbachia pipientis is a commensal microorganism common in insects, including several mosquito species [48]. Interestingly, naturally occurring strains of W. pipientis can increase resistance to various insect borne pathogens such as West Nile virus [49]. While Anopheline mosquitoes are not naturally infected by Wolbachia, Bian and 13 colleagues reported the introduction of a stable Wolbachia infection in the malaria mosquito An. stephensi, conferring resistance to P. falciparum [50]. Attempts to infect An. gambiae with Wolbachia have developed transient somatic infections, but not stable infections [51]. The natural midgut bacterial flora of An. gambiae have been demonstrated to have a protective role against Plasmodium infections. Dong and colleagues determined that mosquitoes cleared of bacteria with antibiotics are more permissive to Plasmodium infection than those with an intact microbiome [52]. Average gut colonization of an An. gambiae mosquito ranges from 104 to 105 bacteria. The Dimopoulos group have studied the bacteria of the An. gambiae microbiome and found significant variation in the quantity and species found in the midgut of mosquitoes deriving from the generation and colony [52]. Nonetheless, certain species are common in an An. gambiae population. Several of these bacteria could indirectly inhibit P. falciparum by activating the mosquito IMD response as previously described [42]. Tchioffo and colleagues determined that the magnitude of Plasmodium inhibition of certain bacterial isolates is greater than other common isolates [53]. Escherichia coli, Serratia marcescens and Pseudomonas stutzeri strains were noted to induce a marked reduction in infection intensity compared with other isolates studied by Tchioffo [53]. The enhanced reduction in infection intensity may be due to certain species being better adapted to colonizing the midgut [53]. Although several bacteria inhibit P. falciparum through activation of the IMD pathway, a strain of Enterobacter is known to actively inhibit P. falciparum [54]. This 14 strain of Enterobacter has been determined to directly inhibit Plasmodium through production of reactive oxygen species. These reactive oxygen molecules inhibit development of P. falciparum in the midgut [54]. In vitro culture of gametocytes with this Enterobacter reduce development to the ookinete stage by approximately 90% [54]. While bacteria have been well studied, other organisms such as fungi and yeast are also present within the midgut. Many studies have focused on bacteria associated with insects, but a number of studies suggest that there may be a large yeast and fungal component of the An. gambiae microbiome. Drosophila, and other plant-associated insects such as leafhoppers have well documented interactions with Candida [55], [56], [57], [58]. Some of these interactions may be only transient, but studies have suggested yeasts may also be permanent components of the respective insects’ microbiomes through interactions with flowers they feed upon [58], [59], [60]. Research in Drosophila by Quintin and colleagues determined that Candida glabrata can persistently infect Drosophila despite Toll immune pathway activation, and it may be that other Candida can similarly avoid clearance by the immune system of insects [61]. Other studies have explored fungi as a potential biocontrol agent. Beauveria bassiana is primarily known as an entomopathogenic bio-control agent. B. bassiana is frequently encountered by An. gambiae through contact with the cuticle of the mosquito. When the fungi settle on the cuticle of a mosquito, they will pierce the cuticle and grow inside the mosquito [62]. Following the death of the insect, B. bassiana spores will be released into the environment from fungal outgrowth on the dead mosquito [62]. 15 This fungus shortens the lifespan of infected Anopheles mosquitoes; Blanford exposed mosquitoes to B. bassiana after providing them a malaria infected bloodmeal, and determined that B. bassiana significantly increases daily mortality in adult mosquitoes over 10 days old. In a cohort of mosquitoes fed a malaria infected blood meal and exposed to B. bassiana, mortality was 65 times higher than a control malaria infected cohort at 11 days post infection [63]. Considering that a mosquito infected with P. falciparum is only infectious to humans after 14 days post malaria infection, this increased mortality reduces the number of mosquitoes that will survive long enough to become infectious [7]. B. bassiana is lethal both to larval and adult An. gambiae, and can be distributed in an oil-based solution for even distribution [64], [65]. Interestingly, mosquitoes resistant to pyrethroid insecticides are more susceptible to B. bassiana [66]. Thus, B. bassiana could be used in place of insecticides, such as pyrethroids, in locations where control is failing. While B. bassiana is a potent mosquito control tool, it must be used carefully, because this fungus does not only infect mosquitoes, but has also a variety of beneficial insects including honey bees and lady beetles [67], [68]. This paper will describe a yeast isolated out of Anopheles gambiae that has an inhibitory effect on Plasmodium falciparum. The yeast is closely related to Candida oleophila (also known as Candida rignihuensis) and Candida railenensis (also known as Apiotrichum osvaldii) [69]. These two yeasts are closely related, and one study by Isaeva posits that they represent varieties of the same anamorphic species with adaptations to different environments [69]. Candida railenensis was isolated in the 1980s from decayed wood and dipterans, and later from the acorns of the English Oak [69], [70], [71]. Considering the variety of 16 locations this yeast and related Candida has been found, including Russia and Chile, it is likely to be ubiquitous across Europe and South America [60], [70], [71], [72]. If the yeast is prevalent in an An. gambiae endemic region, it is likely that the mosquito would have been exposed by landing on spore covered vegetation or by feeding on contaminated plant nectar [73]. C. oleophila has been used as an agricultural biocontrol agent, to protect fruit from harmful Penicillium species. The yeast has activity against fungi, such as Penicillium digitatum, Penicillium italicum, and Geotrichum candidum that can infect wounds on fruit [74]. C. oleophila is known to protect both the fruit as well as the tree against post-harvest diseases [74]. One of the most studied strains is C. oleophila Montrocher, used in the commercial pesticide Aspire [75]. C. oleophila produces lytic enzymes which are highly effective at damaging Penicillium molds; degrading the structural integrity of the fungal cell wall [74], [75]. Several examples of yeast from different genera including C. oleophila are known to produce Exo-β-1, 3-glucanase and chitinase [75], [76]. Other studies have described C. oleophila degrading phenolic compounds and azo dyes found in wastewater [77], [78]. The variety of enzymes secreted by C. oleophila indicates that this yeast can degrade a wide variety of organic compounds. If our isolated species has similar enzymatic activity, it would be interesting to determine whether it degrades protozoal or bacterial cell components. 17 Methods Rearing Anopheles gambiae Mosquitoes An. gambiae, Keele strain mosquitoes were raised and maintained in the Johns Hopkins Bloomberg School of Public Health Insect Core Facility. Mosquitoes were kept in temperature controlled chambers at 27°C and 70% humidity and light cycles inside the chamber were set to 12 hours light/dark. Mosquito feeding was accomplished with a 10% sucrose solution on sterile cotton. If required by the experiment, mosquitoes were transferred to small sterile cups and covered with sterile netting using an aspirator. Yeast Isolates Midguts were dissected from twelve newly emerged female mosquitoes using sterile technique: Prior to dissection, mosquitoes were placed in 100% ethanol for 10 seconds, and rinsed in two consecutive 1x Dulbecco’s PBS baths for a total of 10 seconds. All midgut dissections were done in 1x PBS, using forceps sterilized in 100% ethanol. Dissected midguts were homogenized in 100µL 1x PBS. The homogenized midgut contents were plated on potato glucose agar with 1µg/mL of amphotericin B (Gibco, Grand Island, New York, USA) to select for yeasts and fungi. Potential yeasts were visualized under bright field microscopy to determine their identity. Yeast colonies were replated to derive pure cultures. Sequencing Once the yeast was grown in pure culture, yeast DNA was isolated using chloramphenicol [79]. Isolated DNA was amplified in a Dyad Disciple PCR system 18 using 26S internal transcribed space sequence primers [80]. Aliquots of the isolated DNA were sequenced by the JHMI Sequencing Core facility. Finch TV software (Geospiza, Seattle, WA, USA) was used to view raw sequencing data and insure sequencing data was not contaminated. DNA sequences were aligned with the NCBI BLAST tool. Mosquito Feeding with Yeast and Supernatant Yeast was grown on potato dextrose agar to develop a pure culture. Once a pure culture was established, yeast were grown in potato dextrose broth or glucose-peptone broth at 30°C for approximately two days before use. Before use, the yeast was spun in a Sorvall Legend RT centrifuge (Thermo Scientific, Waltham, MA, USA) at 2000X for 10 minutes. In live yeast experiments, the supernatant is removed and the yeast washed at 2000X for 10 minutes in sterile PBS. The PBS was then removed and the yeast was resuspended in 1mL of 10% sucrose, and fed to Anopheles gambiae on a sterile cotton pad. During supernatant feeding experiments, yeast was grown for three days in potato dextrose broth, and 1.5 mL of the supernatant was passed through a 0.2 µm filter. The resultant filtrate was then mixed with 10% sucrose solution and fed to mosquitoes 3-4 days old at initiation of the experiment on a sterile cotton pad. On day 2 of the experiment, fresh sucrose-supernatant mixture was provided. Plasmodium Challenge The morning of infection with P. falciparum, female mosquitoes that had undergone yeast or supernatant feeding for 2 days were fed on membrane feeders for 30 minutes with NF54W strain gametocytes at 37°C. The gametocyte culture was 19 centrifuged and mixed with red blood cells and serum to the required dilution. Within the first day after feeding, mosquitoes were anesthetized and unfed mosquitoes removed. Engorged mosquitoes were maintained to 8 days post infection. At 8 days post infection, engorged mosquitoes were dissected and their midguts stained with 0.1% mercurochrome. Oocyst numbers were ascertained using a light microscope (Olympus, Center Valley, PA, USA), and the median was calculated. Quantification of Midgut Bacteria Quantification of midgut bacteria was assayed by the colony forming unit assay. Mosquitoes were dissected using sterile technique, and their midguts homogenized. Serial dilutions of midgut homogenate were carried out in sterile PBS and 75 µl of each dilution were plated on LB media. Ten-fold dilutions were carried out to a final dilution of 1x10-7. The agar plates were incubated at 20°C for three days prior to counting. When assessing which dilution to count, the dilution closest to the 20-250 colony forming unit range was chosen. Plate counts were converted to total numbers of bacteria per midgut and log10 transformed using Microsoft Excel. In- vitro P. berghei ookinete inhibition assay To test the inhibition of ookinete development by C. oleophila, yeast were cocultured with P. berghei gametocytes in an in vitro system. Six to eight week old female Swiss-Webster mice were infected with a transgenic P. berghei strain (PbwRL) expressing a Renilla luciferase controlled by the WARP promoter, an ookinete specific promoter [81]. The supernatant from C. oleophila was removed, and the yeast used in the experiment were washed twice in sterile PBS for ten minutes at 2000 rpm. Yeast was 20 prepared at concentrations of 1x105, 1x107, and 1x109 yeast cells per 50 µL of sterile PBS. Ookinetes were incubated at 19 C for 26-28 hours in culture with yeast solutions at either 1x105, 1x107, or 1x109 yeast per 50 µL. The resultant culture was transferred to a 1.5 mL Eppendorf and spun at 1800 r.p.m. for 4 minutes [81]. The supernatant was discarded following centrifugation [81]. The Renilla luciferase system (E2810, Promega) was used to perform the luciferase assay, following the manufacturer’s instructions (G. Mlambo and G. Dimopoulos, unpublished) [81]. To summarize, 100μl of 1X lysis buffer was added the sample and vortexed gently [81]. The solution was incubated at 20°C for 15 min. The lysate was centrifuged at 14000 r.p.m. for 2 min, and the supernatant was transferred to a 1.5 ml Eppendorf tube and placed on ice [81]. A total of 20 µL of supernatant was mixed by pipette into 100μl of luciferase assay buffer [81]. The resultant solution was immediately read on a 20/20n Luminometer (Turner Biosystems, Sunnyvale, CA, USA) over a 10 sec integration period [81]. During each experiment, three technical replicates were performed and the output rendered in arbitrary luminescence units. Four biological replicates were performed. Longevity Assay Three to four day old female mosquitoes were sorted into two groups of at least 40, and fed either on a control 10% sucrose solution or on live yeast in 10% sucrose solution prepared as described. Cohorts were kept separate in small, sterile wax-coated cups in conditions as seen above in Rearing Mosquitoes section of the methods. The second day, fresh yeast was provided to the test group. On the third day and afterward, the mosquitoes were maintained on 10% sucrose solution. Counts of dead mosquitoes 21 were made each day at a fixed time, until all mosquitoes died. Six biological replicates were performed. Data analysis All data analysis was done with Graphpad Prism 5 software (GraphPad Software, La Jolla, CA, USA) 22 Results Isolation and Sequencing of C. oleophila A previous ScM student of the Dimopoulos group had isolated a yeast from the Insect Core Facility An. gambiae colony. We determined that the same yeast is still present in the Insect Core’s An. gambiae population through sequencing. The ribosomal 26S internal transcribed space sequence of yeasts is a region removed prior to the production of mature rRNA in the yeast. Internally transcribed spacers are frequently used in identification of fungal and yeast species, and we were able to amplify this region and determine its nucleotide sequence identity [82]. No reference sequence completely matched the amplified 26S sequence in NCBI database. BLAST sequence similarity analysis indicate this yeast is closely related to Candida oleophila and Candida railenensis, two yeasts which literature indicates are members of the same anamorphous yeast species [69]. Based on BLAST results, we are considering this yeast a strain of C. oleophila. The Presence of C. oleophila in the Mosquito Midgut Inhibits P. falciparum Infection. To test whether our Candida isolate could influence An. gambiae susceptibility to P. falciparum infection, we first introduced it into the mosquito midgut through sucrose feeding and then fed the mosquitoes on a parasite gametocyte culture. Mosquitoes were fed approximately 1x 109 live C. oleophila spores in a 10% sucrose solution for two days, while control mosquitoes received a 10% sucrose solution devoid of yeast spores. On the third day mosquitoes were fed on a P. falciparum gametocyte culture and 23 infection intensity and prevalence was determined 7 days later by counting oocyst on dissected midguts. Those mosquitoes that harbored C. oleophila displayed a significant reduction in the intensity of P. falciparum infection. The control group showed a median infection intensity of 13 oocysts, while the yeast fed group had a median of 9 oocysts on the midgut (Figure 5). The control group displayed a much greater range of oocysts on the midgut than the yeast fed groups: the oocyst range of the control was 0-50 oocysts, while the yeast group had a range of 0-34 oocysts per midgut. This indicates C. oleophila inhibits a pre-oocyst stage of P. falciparum in the mosquito midgut. Not only did the presence of the yeast reduce the quantity of oocysts in those midguts that were infected, but also reduced infection prevalence. The control group had a prevalence of approximately 97% which was reduced down to approximately 91% in the Candida-fed group. We then wanted to determine whether the Plasmodium life cycle was inhibited by some factor secreted by the yeast, or whether the yeast spore had to be present for inhibition. 24 oocysts/midgut 60 ** 40 20 C .O le co nt ro l op hi la 0 Figure 5. The impact of C. oleophila presence in the midgut on P. falciparum oocyst development in vivo. Horizontal lines indicate the median infection intensity. A MannWhitney test was used to determine statistically significant difference with a P value of 0.0076. Oocyst Development is Inhibited in the Mosquito Midgut by a C. oleophila -Secreted Factor Once the C. oleophila was determined to inhibit the development of oocysts in the midgut, we wanted to test whether inhibition depended on the presence of whole yeast spores in the midgut or a yeast secreted factor. Literature indicates that C. oleophila secretes multiple enzymes to degrade organic compounds in its environment [74], [77], [78]. By filtering a yeast culture supernatant through a 0.2 micron filter, we could ensure that the resultant filtrate would not contain whole yeast cells, but only yeast secreted factors. After feeding mosquitoes for two days on the culture supernatant filtrate in a 10% 25 sucrose solution, we infected them with P. falciparum as described above and found significantly reduced oocyst numbers compared to the control group that received a 10% sucrose solution without the yeast supernatant filtrate. The range of oocysts on the midgut was also reduced in those mosquitoes that had received filtered yeast supernatant. The mosquitoes that had fed on the yeast culture filtrate contained a range of 0 to 21 oocysts per midgut, while the control group contained 0 to 48 oocysts per midgut. The median number of oocysts per midgut was 10 and 4 in the control and experimental groups, respectively. The prevalence was also reduced from 89% to 83% in the yeast culture filtrate fed group. This data suggests that some yeast-secreted factor is produced by the yeast that inhibits the development of the P. falciparum. 60 oocysts/midgut ** 40 20 C .O le op hi la co nt ro l 0 Figure 6. C. oleophila culture supernatant filtrate inhibits development of oocysts in An. gambiae. Horizontal lines indicate the median oocyst infection intensity. The asterisks indicate statistical significance at a p value of 0.0035 when using the Mann-Whitney test. 26 C. oleophila Does Not Inhibit Plasmodium berghei Ookinete Development in vitro. C. oleophila inhibits P. falciparum development in vivo, but the specific stage of the parasite that was inhibited was unknown. To determine which stage of Plasmodium the yeast acted on, we utilized an in vitro P. berghei ookinete culture assay in conjunction with yeast exposure. Exposure of the ookinete culture to different concentrations of live yeast would indicate whether the yeast would inhibit the development of a pre-ookinete stage. Following three trials it was concluded that there is no significant difference in development of ookinetes between groups exposed to sterile PBS and those exposed to yeast. In each concentration of 105, 107 and 109 C. oleophila per 50 microliter ookinete culture there was no significant difference between the treated and control groups. Exposure of the ookinete culture to a 105 C. oleophila per 50 microliter ookinete culture resulted in an increased development of ookinetes, compared to the control (fig 7). Florescence Units 1500000.0 1000000.0 500000.0 10 ^9 10 ^7 10 ^5 ct rl 0.0 Spores/50L Figure 7. There is no significant difference in ookinete development as concentration of C. oleophila spores increases. There was no significant difference between groups with a P value of 0.3916 according to the Kruskal-Wallace test. 27 Presence of C. oleophila Results in an Increased Bacterial Load of the Mosquito Midgut We were interested in assaying the influence of the yeast on the bacteria of the mosquito midgut. The colony forming unit assay measures the number of live aerobic bacteria present in a sample. In this experiment, we plated the contents of homogenized midguts, from either yeast fed or control mosquitoes, on LB agar petri dish plates and counted bacterial colonies on the plates after 3 days at room temperature. Mosquitoes fed with Candida yeast show an increased aerobic bacterial load compared to the control mosquitoes that had fed on a 10% sucrose solution. The log10 transformed median number of bacteria per midgut in the control group was 3.5 whereas the log10 median number of bacteria per midgut of the yeast-fed group was 4.6. Additionally, the phenotypes of the bacteria plated from yeast harboring midguts suggests that the presence of this yeast in the midgut may alter the prevalence of several bacterial species within the midgut. The identity of those species has not yet been determined. 28 Ingesting C. Oleophila increases the number of midgut bacteria ** Log10 CFU per Midgut 6 4 2 op hi la .O le C C on tr ol 0 Figure 8. The presence of C. oleophila in the An. gambiae midgut increases the bacteria load of the midgut. Asterisks indicate statistical significance of P<0.01. The two groups differ with a value of P =0.0081 according to the Mann-Whitney test. The Presence of C. oleophila in the Midgut Does Not Influence Mosquito Longevity We hypothesized that the increased bacterial load of the yeast-containing midgut would result in an immune activation that could be costly to the mosquito and therefore result in decreased longevity. The longevity assay is commonly used to assess the effects of an experimental treatment on the mosquito lifespan. Groups of 40 mosquitoes were fed on either a 10% sucrose solution or a 10% sucrose solution containing 1x109 C. oleophila spores for two days. After two days, the mosquitoes were maintained on a 10% sucrose solution and dead mosquitoes were counted at the end of each day. Throughout the course of the longevity assay, mosquitoes of the two groups died at approximately the same rate (fig 9). 29 Percent survival 100 control yeast 80 60 40 20 0 0 5 10 15 Day Figure 9. There is no significant difference between control and yeast fed mosquitoes survival. The Gehan-Breslow-Wilcoxen test determined a P value of 0.2483. Median survival in the yeast fed group was 11 days compared to 10 days in the control group. Discussion 30 This study has successfully isolated a yeast likely to be a subspecies of C. oleophila from the midgut of the P. falciparum vector An. gambiae. C. oleophila is a biocontrol organism used in agriculture to protect crops from Penicilium molds [74]. It is frequently used post-harvest to reduce infection by P. digitatum among other fungi [74]. Our C. oleophila isolate displays interesting properties in the malaria mosquito An. gambiae when introduced through the oral route. Because this yeast was originally isolated from the midgut of An. gambiae, we wanted to assess its effect on the lifecycle of the mosquito and the malaria parasite P. falciparum. We used a natural ingestion route in our assays; mosquitoes in the field are most likely to encounter yeast through feeding on contaminated nectar sources [73]. When fed to An. gambiae two days prior to Plasmodium infection, the yeast significantly inhibits P. falciparum oocyst development in the midgut. Both the intensity and prevalence of oocysts were reduced in yeast fed mosquitoes compared to the control. However, the yeast does not inhibit in vitro P. berghei ookinete development significantly, suggesting that C. oleophila does not prevent development from the gametocyte to ookinete. Inhibition most likely occurs at the pre-oocyst stage, either as the ookinete binds to the midgut epithelium or as it traverses the midgut. Alternatively, the yeast may induce a mosquito response that acts against the parasite [53], [54]. Another possibility is that the presence of yeast induces an altered midgut bacterial microflora that would reduce Plasmodium infection [53], [54]. The effects of this yeast in vivo are especially interesting when considering the yeast’s potential negative effects on the ookinete and early oocysts. This Candida may be secreting various glucanases and proteases that could degrade ookinete surface proteins. 31 The yeast in this study is related to C. oleophila, which is known to secrete a variety of enzymes including proteases and glucanases and also produces enzymes that degrade Azo dyes and phenolic compounds [74], [75], [76], [77], [78]. If the Candida species produces proteases while in the midgut, those secreted enzymes may interact with the ookinete and prevent the ookinete from continuing on to the oocyst stage. If ookinete surface proteins are cleaved, an ookinete may not be able to bind to the midgut in order to carry out the process of forming an oocyst. Alternatively, yeast could prevent the ookinetes from reaching the midgut epithelium through steric hindrance. In order to better understand the interactions of the yeast with An. gambiae, quantification of C. oleophila through real time PCR using the 26S Internal Transcribed Spacer sequence could be used whether it expands following ingestion or whether it is cleared from the midgut [80]. An anti-C. oleophila antibody could give insight into the interactions of the yeast within the midgut and malaria parasite through co-localization. However, our assays with yeast culture supernatant filtrates suggest that a co-localization is not necessary for Plasmodium inhibition. Mosquito midguts harboring the Candida yeast show a significant increase in their bacterial load compared to the controls. In vitro yeast and bacterial co-culture experiments could provide insights on whether the yeast provides a bacterial growth advantage by providing nutrients, or whether the increased bacterial load is because of a mosquito-mediated process [37]. It has been shown that P. falciparum inhibition by bacteria is dose-dependent, so if bacterial populations expand following introduction of C. oleophila, this could be sufficient to inhibit P. falciparum [81]. If the mosquito is host to bacteria that produce anti-parasitic compounds, the presence of the yeast may enhance 32 the effect of those organisms, thereby resulting in Plasmodium inhibition [54]. The results suggest a bacteria-mediated mechanism of action is likely. If the yeast-mediated bacterial expansion is proven to be responsible, the specific bacterial species that proliferate in the presence of the yeast should be explored. This could be performed through a metagenomic analysis of C. oleophila containing and control mosquito midguts. Quantitative real-time PCR with primers specific to a given bacterial species could also be used to determine whether any of the species in the midgut are expanding [84]. The role of mosquito-associated fungi and yeast has to date not been thoroughly studied. The Favia lab has studied the yeast Wickerhamomyces anomalus (formerly Pichia anomala) in the mosquito Anopheles stephensi, and its potential for paratransgenesis. Favia’s study could direct the future study of C. oleophila, since W. anomalus is found in several compartments of the mosquito including the female reproductive tract, suggesting that it may be useful to investigate whether Candida species is present in the Malpighian tubules, ovaries, as well as the hemolymph [80]. Our study has shown the possibility of this yeast as a biocontrol agent, and more work must be done to determine the nature of its interaction with Anopheles and Plasmodium. The Johns Hopkins An. gambiae strain that has been kept in the laboratory environment for hundreds of generations, and we can therefore not be certain whether its interaction with C. oleophila reflects field mosquito-yeast interactions. The study of yeast in natural field An. gambiae should therefore provide a better understanding of how the yeast interacts with the mosquito. The magnitude of C. oleophila mediated P. falciparum 33 inhibition may differ in mosquitoes exposed to a natural environment as opposed to a controlled laboratory environment. One could speculate that the inhibition may vary between different An. gambiae strains due to genetic variations in immune pathways, as well as differences of their microbiomes [85], [86]. However, our study represents an important first step towards a better understanding of yeast mosquito interactions, and how these can influence vector competence. Our C. oleophila strain inhibits P. falciparum in vivo and alters the bacteria load of the midgut. Isolation and characterization of C. oleophila strains from wild mosquitoes would provide insight into the anti-Plasmodium properties of different strains of this yeast. It is still unknown how the C. oleophila strain isolated in this study was introduced into the mosquitoes of the insectary. While this particular yeast species has not been reported in wild An. gambiae, it has been identified at locations in both South America and Eurasia [69], [72]. It is possible that this yeast is a ubiquitous member of the Anopheles microbiome across Africa, and that it influences Plasmodium transmission to some degree. This is also true for a variety of other microorganisms that have been shown in the laboratory to have an inhibitory effect on P. falciparum [52], [53], [54], [81]. Most studies concerning malaria control using fungi have concentrated on entomopathogenic properties but not in any great detail on Plasmodium inhibitory properties [63], [64], [65], [66]. The role of fungi and yeast as commensal organisms through the life span of the mosquito has not been explored in detail, but should be investigated thoroughly to better understand the microbiome. Fungi are ubiquitous in the environment where mosquitoes are present, and non-entomopathogenic fungi may come 34 into contact with mosquitoes through various routes [60]. Even if these organisms may only interact transiently with the mosquito, it is still important to investigate whether they cause mosquito immune modulation. Further studies are also needed to better understand the anti-Plasmodium activity of C. oleophila isolate in the context of vector competence. In this study, mosquitoes were infected with P. falciparum two days after they had fed on the yeast, and it would be interesting to determine how long this anti-Plasmodium effect lasts after the mosquito ingests the Candida spores. The life span of an An. gambiae mosquito is relatively short, with the majority of laboratory-reared mosquitoes surviving up to a month [9]. At field conditions, mosquito lifespan is shorter due to limited food supply, predators, and microbes such as entomopathogenic fungi [7]. Considering that a mosquito becomes infectious two weeks after consuming P. falciparum gametocytes, most infectious mosquitoes are close to reaching their life expectancy [5], [8], [9]. If a mosquito is delayed by several days in becoming competent to support Plasmodium infection, the risk of it transmitting the disease would be reduced. Even if the mosquito only feeds once on C. oleophila in the field, it may still become protected from P. falciparum infection for several days afterward. To further elucidate the duration of C. oleophila anti-Plasmodium effects, mosquitoes could be yeast fed 3, 5, or 7 days prior to feeding on a gametocyte culture. While the approximately 5% reduction in infected mosquitoes compared to control may not significantly impact mosquito vector competence in a lab setting, infection intensity is much lower in nature than in a controlled laboratory infection assay. In natural settings, less than 5 oocysts will typically develop after an infectious bloodmeal. 35 In a study done by Taylor, An. gambiae contained on average 3.38 oocysts in their midgut, and only 11% of mosquitoes that had fed on infected blood had become infected [86]. In our study, over half of mosquitoes fed to repletion on infected blood and the over 80% percent of the control group develop infections. It would be interesting to determine whether the presence of C. oleophila in natural conditions described by Taylor could clear An. gambiae of P. falciparum infection. Guerra and colleagues studied the global intensity of transmission, and suggest that outside of Africa most transmission is hypoendemic [87]. In the past decade, antimalarial drugs and other control strategies have become widely available [88], [89], [90]. In areas of low endemicity, a dedicated effort using multiple control strategies could realistically eliminate P. falciparum [87]. This yeast is it is very inexpensive to culture and it could therefore be developed into a low-cost malaria control strategy to supplement existing malaria control strategies. While promising, this yeast requires additional study. We have not yet investigated whether this C. oleophila strain can be vertically transmitted from parent to offspring, or whether it would require continuous application in the field for mosquito exposure. If this yeast is deployed to the field, it will take its place alongside other malaria interventions. Current treatments have been very successful in reducing the morbidity and mortality from P. falciparum infection, but Plasmodium infection remains a major public health threat [18], [88], [89], [90]. Introducing an anti-Plasmodium microbe as part of an integrated control strategy involving drug treatment and insecticide treated bednets would 36 leverage its effectiveness to make a significant reduction in malaria infection [54], [88], [91]. This is the first study to investigate C. oleophila as a component of the An. gambiae microbiome, and discovery of its anti-Plasmodium properties highlights the need to explore the yeast and fungi populations of An. gambiae in greater depth. References 37 1)Cunha C B.,Cunha B.A. 2008. Brief history of the clinical diagnosis of malaria: from Hippocrates to Osler. J Vector Borne Dis 45:194–199 2)Cox FEG 2010. History of the discovery of the malaria parasites and their vectors. Parasites & Vectors 3:5 3)Snow RW, Guerra CA, Noor AM, Myint HY, and Hay SI. 2005.The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 434(7030): 214–217. 4)Malaria Atlas Project. 2007. The Clinical Burden of Plasmodium falciparum. http://www.map.ox.ac.uk/browse-resources/clinical-burden/Pf_burden/world/ 5)Gillies M.T., and Coetzee M.1987. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Publications of the South African Institute for Medical Research 55: 1–143 6)Malaria Vaccine Initiative. 2004. Fact Sheet: Plasmodium falciparum malaria. http://www.malariavaccine.org/files/FS_Pfalciparum-Sept-2004_FINAL.pdf 7)Ghosh, A., Edwards, M.J., and Jacobs-Lorena, M. 2000.The journey of the malaria parasite in the mosquito: hopes for the new century. Parasitol Today 16:196–201 8) CDC. 2010. Biology in About Malaria. http://www.cdc.gov/malaria/about/biology/ 9)Smith, D. L., & McKenzie, F. E. (2004). Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria Journal, 3(1), 13. 10) Vaughan, J. A., Noden, B. H., & Beier, J. C. (1992). Population dynamics of Plasmodium falciparum sporogony in laboratory-infected Anopheles gambiae. The Journal of parasitology, 78(4), 716-724. 11)Tsy, J. M. L. P., Duchemin, J. B., Marrama, L., Rabarison, P., Le Goff, G., Rajaonarivelo, V., & Robert, V. (2003). Distribution of the species of the Anopheles gambiae complex and first evidence of Anopheles merus as a malaria vector in Madagascar. Malaria journal, 2(1), 33. 12)Gimonneau G, Pombi M, Choisy M, Morand S, Dabire RK, et al. (2012) Larval habitat segregation between the molecular forms of the mosquito Anopheles gambiae in a rice field area of Burkina Faso, West Africa. Medical and Veterinary Entomology 26: 9–17. 13) Lindsay SW, Parson L, and Thomas CJ.1998. Mapping the ranges and relative abundance of the two principal African malaria vectors,Anopheles gambiae sensu stricto and An. arabiensis, using climate data. Proc. R. Soc. Lond. B 265(1399):847-854 14)Githeko AK, Ototo EN, and Guiyun Y 2012.Progress towards understanding the ecology and epidemiology of malaria in the western Kenya highlands: opportunities and challenges for control under climate change risk. Acta Trop. 121(1): 19–25. 15)Despommier D, Gwadz R, Hotez P, Knirsch C (Ed.).2005.Summary of Basic Science and Clinical Information for Malaria in Parasitic Diseases 5th Edition. Apple Trees Productions 16)Lozano, R., Lopez, A. D., Atkinson, C., Naghavi, M., Flaxman, A. D., & Murray, C. J. (2011). Performance of physician-certified verbal autopsies: multisite validation study using clinical diagnostic gold standards. Popul Health Metr, 9, 32. 38 17) Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet. 379, (9814):413–431 18) WHO: World Malaria Report (2013). Geneva: World Health Organization;2013. 19)Utzinger, J., Tozan, Y., & Singer, B. H. (2001). Efficacy and cost‐effectiveness of environmental management for malaria control. Tropical Medicine & International Health, 6(9), 677-687. 20)White N. J. 2008. Qinghaosu (Artemisinin):The Price of Success.Science :320(5874):330-4. 21)Trape J, Tall A, Diagne N, Ndiath O, Ly AB, Faye J, Dieye-Ba F, Roucher C, Bouganali C, Badiane A, Sarr FD, Mazenot C, Touré-Baldé A, Raoult D, Druilhe P, Mercereau-Puijalon O, Rogier C, Sokhna C, 2011. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis 11 (12):925-932 22)Keiser, J., Singer, B. H., & Utzinger, J. (2005). Reducing the burden of malaria in different ecoepidemiological settings with environmental management: a systematic review. The Lancet infectious diseases, 5(11), 695-708. 23)Krafts, K., Hempelmann, E., & Skórska-Stania, A. (2012). From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy. Parasitology research, 111(1), 1 24)Frosch AEP, Venkatesan M, and Laufer MK. 2011. Patterns of chloroquine use and resistance in subSaharan Africa: a systematic review of household survey and molecular data. Malaria Journal 10:116 25)World Health Organization (2010) Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization 26) Cohen JL, Yadav P, Moucheraud C, Alphs S, Larson PS, et al. (2013) Do Price Subsidies on Artemisinin Combination Therapy for Malaria Increase Household Use?: Evidence from a Repeated CrossSectional Study in Remote Regions of Tanzania. PLoS ONE 8(7): e70713 27) Rehwagen, C. (2006). WHO ultimatum on artemisinin monotherapy is showing results. BMJ, 332(7551), 1176. 28)Abdulla S, Salim N, Machera F, Kamata R, Juma O, Shomari M,Kubhoja S,Mohammed A, Mwangoka G, Aebi T, Mshinda H, Schellenberg D,Carter TVillafana T, Dubois MC, Leach A, Lievens M, Vekemans J,Cohen J, Ballou WR, and Tanner M. 2013. Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS,S/AS02D malaria vaccine in infants living in a malaria-endemic region. Malaria Journal 12:11 29) Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z and Corbel V. 2011. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends in Parasitology 27(2):91-98 30)Wondji CS, Coleman M, Kleinschmidt I, Mzilahowa T, Irving H, Ndula M, Rehman A, Morgan J, Barnes KG, Hemingway J. 2012. Impact of pyrethroid resistance on operational malaria control in Malawi. PNAS 109(47):19063-19070 31) Corbel V and N’Guessan R. 2013. Distribution, Mechanisms, Impact and Management of Insecticide Resistance in Malaria Vectors: A Pragmatic Review, Anopheles mosquitoes - New insights into malaria vectors, Prof. Sylvie Manguin (Ed.). 39 32) Trape J, Tall A, Diagne N, Ndiath O, Ly AB, Faye J, Dieye-Ba F, Roucher C, Bouganali C, Badiane A, Sarr FD, Mazenot C, Touré-Baldé A, Raoult D, Druilhe P, Mercereau-Puijalon O, Rogier C, Sokhna C, 2011. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis 11 (12):925-932 33)Gnankiné O,Bassolé IHN,Chandre F, Glitho I, Akogbeto M, Dabiré RK, Martin T. 2013. Insecticide resistance in Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) and Anopheles gambiae Giles (Diptera: Culicidae) could compromise the sustainability of malaria vector control strategies in West Africa. Acta Tropica 128 (1): 7–17 34)Wang S, Jacobs-Lorena M .2013.Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends in Biotechnology 31(3):185-193 35)Ghosh A, Srinivasan P, Abraham EG, Fujioka H, and Jacobs-Lorena M. 2003. Molecular strategies to study Plasmodium– mosquito interactions. Trends in Parasitology 19(2)::94-101 36)Gardner MJ, Hall N, Fung E, Owen White, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan M, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DMA, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Gettins PG 2002. Serpin structure, mechanism, and function. Chem Rev 102 (12): 4751–804. 37) Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong Y, Dimopoulos G. 2012. Anopheles Imd Pathway Factors and Effectors in Infection Intensity-Dependent Anti-Plasmodium Action. PLoS pathogens, 8(6), e1002737. 38) Volz J, Müller HM, Zdanowicz A, Kafatos FC, and Osta MA.2006. A genetic module regulates the melanization response of Anopheles to Plasmodium Cell. Microbiol 8(9):1392–1405 39)Hernandez-Martinez S, Lanz H, Rodriguez MH, Gonzalez-Ceron L, and Tsutsumi V.2002. CellularMediated Reactions to Foreign Organisms Inoculated into the Hemocoel of Anopheles albimanus (Diptera: Culicidae) J. Med. Entomol. 39(1): 61Ð69 40)Castillo JC, Robertson AE, Strand MR.2006. Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochemistry and Molecular Biology 36(12): 891–903 41)Meister S, Kanzok SM, Zheng XL, Luna C, Li TR Hoa NT,Clayton JR, White K Kafatos FC , Christophides GK and Zheng L. 2005. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. PNAS 102 (32): 1420–11425 41)Rodrigues, J., Brayner, F. A., Alves, L. C., Dixit, R., & Barillas-Mury, C. (2010). Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science, 329(5997), 1353-1355. 42) Garver LS, Dong Y, Dimopoulos G (2009) Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog 5: e1000335. doi: 10.1371/journal.ppat.1000335 43) Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. (1996) The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults Cell 86 , 973-983. 44)Michel, T., Reichhart, J. M., Hoffmann, J. A. & Royet, J. (2001). Drosophila Toll is activated by Grampositive bacteria through a circulating peptidoglycan recognition protein. Nature 414 , 756-759 40 45)Garver LS, de Almeida Oliveira G, Barillas-Mury C (2013) The JNK Pathway Is a Key Mediator of Anopheles gambiae Antiplasmodial Immunity. PLoS Pathog 9(9): e1003622. 46)Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, et al. (2009) The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 5: 498– 507. 47)Dillon RJ, Dillon VM. 2004.The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49: 71–92. 48)Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia? - a statistical analysis of current data. FEMS Microbiol Lett 281: 215–220. 49) Glaser RL, Meola MA (2010) The Native Wolbachia Endosymbionts of Drosophila melanogaster and Culex quinquefasciatus Increase Host Resistance to West Nile Virus Infection. PLoS ONE 5(8): e11977. 50) Bian, G., Joshi, D., Dong, Y., Lu, P., Zhou, G., Pan, X., ... & Xi, Z. (2013). Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science, 340(6133), 748-751. 51) Kambris Z, Blagborough AM, Pinto SB, Blagrove MSC, Godfray HCJ, et al. (2010) Wolbachia Stimulates Immune Gene Expression and Inhibits Plasmodium Development in Anopheles gambiae. PLoS Pathog 6(10): e1001143. 52) Dong Y, Manfredini F, Dimopoulos G (2009) Implication of the Mosquito Midgut Microbiota in the Defense against Malaria Parasites. PLoS Pathog 5(5): e1000423. 53) Tchioffo MT, Boissière A, Churcher TS, Abate L, Gimonneau G, et al. (2013) Modulation of Malaria Infection in Anopheles gambiae Mosquitoes Exposed to Natural Midgut Bacteria. PLoS ONE 8(12): e81663 54) Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, Dimopoulos G. 2011. Natural Microbe-Mediated Refractoriness to Plasmodium Infection in Anopheles gambiae. Science 332 (6031):855-858 55) Pang K, Dong S, Hou Y, Bian Y, Yang K, Yu XP.2012 Cultivation, identification and quantification of one species of yeast-like symbiotes, Candida, in the rice brown planthopper, Nilaparvata lugens. Insect Science 19(4):477-484. 56) Dong S, Pang K,Bai X,Yu XP, Hao PY. 2011. Identification of Two Species of Yeast-like Symbiotes in the Brown Planthopper Nilaparvata lugens. Curr microbiology 62:1133-1138 57) Suh S,Nguyen NH, Blackwell M.2005. Nine new Candida species near C. membranifaciens isolated from insects. Mycological Research 109(9):1045-1056 58) Morais PB, Hagler AN, Rosa CA, Mendonca-Hagler LC, Klaczko LB. 1992. Yeasts associated with Drosophila in tropical forests of Rio de Janeiro, Brazil. Can J Microbiol. 38(11):1150-5. 59)Lachance MA, Bowles JM, Chavarría Díaz MM, and Janzen DH. 2001. Candida cleridarum, Candida tilneyi and Candida powellii, three new yeast species isolated from insects associated with flowers. IJSEM 51(3):1201-1207 41 60) Serjeant K, Tang R, Anfang N, Beggs JR, and Goddard MR .2008 Yeasts associated with the New Zealand Nothofagus honeydew system. New Zealand Journal of Ecology32(2):209-213 61) Quintin J, Asmar J, Matskevich AA, Lafarge MC, Ferrandon D. 2013. The Drosophila Toll pathway controls but does not clear Candida glabrata infections. The Journal of Immunology 190 (6): 2818-2827 62) Madelin M. F.(1963). Diseases caused by hyphomycetous fungi. “Insect Pathology-An Advanced Treatise” (Steinhaus, E. A., ed.), 2: 233- 271. Academic Press, New York. 63)Blanford, S., Chan, B. H., Jenkins, N., Sim, D., Turner, R. J., Read, A. F., & Thomas, M. B. (2005). Fungal pathogen reduces potential for malaria transmission. Science, 308(5728), 1638-1641. 64) Bukhari T, Middelman A, Koenraadt CJM, Takken W, Knols BGJ (2010) Factors affecting fungus-induced larval mortality in Anopheles gambiae And Anopheles stephensi. Malaria J. 9 :22 65)Mnyone, L. L., Kirby, M. J., Lwetoijera, D. W., Mpingwa, M. W., Simfukwe, E. T., Knols, B. G., ... & Russell, T. L. (2010). Tools for delivering entomopathogenic fungi to malaria mosquitoes: effects of delivery surfaces on fungal efficacy and persistence. Malar J, 9, 246. 66)Howard AF, Koenraadt CJ, Farenhorst M, Knols BG, Takken W. Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malar J. 2010a;9:168. 67)Shipp, L., Kapongo, J. P., Park, H. H., & Kevan, P. (2012). Effect of bee-vectored Beauveria bassiana on greenhouse beneficials under greenhouse cage conditions. Biological Control, 63(2), 135-142. 68)R.R. James, B. Lighthart.(1994). Susceptibility of the convergent lady beetle (Coleoptera: Coccinellidae) to four entomopathogenic fungi. Environmental Entomology, 23 :190–192 69) Isaeva O.V., Glushakova A.M., Yurkov A.M., and Chernov IY.2009.The Yeast Candida railenensis in the Fruits of English Oak (Quercus robur L.) Microbiology 78(3):399-403 70) Glushakova A.M., Yurkov A.M., and Chernov IY 2007. Massive Isolation of Anamorphous Ascomycete Yeasts Candida oleophila from Plant Phyllosphere. Microbiology, 76(6), 799-803. 71) Ramírez C. & González A.E. 1984. Candida railenensis. Mycopathologia, 88 (1): 55 72)Pimenta RS, Alves PDD, Almeida GMF, Silva JFM, Morais PB, Corrêa A; Rosa CA. 2009.Yeast communities in two Atlantic rain Forest fragments in Southeast Brazil. Braz. J. Microbiol. 40 (1):90-95. 73) Gary RE Jr, Foster WA. 2004.Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Med Vet Entomol 18: 102–107 74)Lahlali R. , SerrhiniM.N., M.H. Jijakli 2004Efficacy Assessment of Candida oleophila(Strain O) and Pichia anomala (STRAIN K)Against Major Postharvest Diseses of Citrus Fruits In Morocco. Comm. Appl. Biol. Sci, 69 (4):601-610 75) Droby S., Vinokur V., Weiss B., Cohen L., Daus A., Goldschmidt E. E., and Porat R. Induction of Resistance to Penicillium digitatum in Grapefruit by the Yeast Biocontrol Agent Candida oleophila. Phytopathology, 92(4), 393-399. 76)Segal E, Yehuda H, Droby S, Wisniewski M, and Goldway M.2002. Cloning and analysis of CoEXG1, a secreted 1,3-β-glucanase of the yeast biocontrol agent Candida oleophila.Yeast 19, (13): 1171–1182 42 77)Lucas, M. S., Amaral, C., Sampaio, A., Peres, J. A., & Dias, A. A. (2006). Biodegradation of the diazo dye Reactive Black 5 by a wild isolate of< i> Candida oleophila</i>. Enzyme and Microbial Technology, 39(1), 51-55. 78)Amaral C, Lucas MS, Sampaio A, Peres JA, Dias AA, Peixoto F, Rosário Anjos M, Pais C. 2012. Biodegradation of olive mill wastewaters by a wild isolate of Candida oleophila. International Biodeterioration and Biodegradation 68 :45-50 79)Logemann, J., Schell, J., & Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Analytical biochemistry, 163(1), 16-20. 80)Ricci I, Damiani C, Scuppa P, Mosca M, Crotti E, Rossi P, Rizzi A, Capone A, Gonella E, Ballarini P, Chouaia B, Sagnon N, Esposito F, Alma A, Mandrioli M, Sacchi L, Bandi C, Daffonchio D, and Favia G. 2012. The yeast Wickerhamomyces anomalus (Pichia anomala) inhabits the midgut and reproductive system of the Asian malaria vector Anopheles stephensi. Curr. Opinion in Micro. 15(3):278–284 81)Bahia, A. C., Dong, Y., Blumberg, B. J., Mlambo, G., Tripathi, A., BenMarzouk-Hidalgo, O. J., Chandra, R. and Dimopoulos, G. (2014), Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environmental Microbiology. 82)Kurtzman, C. P., & Robnett, C. J. (1998). Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek, 73(4), 331-371. 84)Makino H, Fujimoto J, Watanabe K.2010. Development and evaluation of a real-time quantitative PCR assay for detection and enumeration of yeasts of public health interest in dairy products. International Journal of Food Microbiology 140,(1) :76–83 85)Weiss, B., & Aksoy, S. (2011). Microbiome influences on insect host vector competence. Trends in parasitology, 27(11), 514-522. 86)Beerntsen BT, James AA, and Christensen BM. 2000. Genetics of Mosquito Vector Competence. Microbiol. Mol. Biol. Rev. 64(1): 115-137 85) Zimmermann, G. (1993). The entomopathogenic fungus Metarhizium anisopliae and its potential as a biocontrol agent. Pesticide Science, 37(4), 375-379. 86) Taylor, L. H. (1999). Infection rates in, and the number of Plasmodium falciparum genotypes carried by Anopheles mosquitoes in Tanzania. Annals of tropical medicine and parasitology, 93(6), 659-662. 87) Guerra, C. A., Gikandi, P. W., Tatem, A. J., Noor, A. M., Smith, D. L., Hay, S. I., & Snow, R. W. (2008). The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS medicine, 5(2), e38. 88)Barat LM (2006) Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam. Am J Trop Med Hyg 74: 12–16. 89) Nyarango PM, Gebremeskel T, Mebrahtu G, Mufunda J, Abdulmumini U, et al. (2006) A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J 5: 33. 90) Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, et al. (2005) Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med 2: e330. 43 91) Cirimotich, C.M., Clayton, A.M., and Dimopoulos, G. (2011). Low- and high-tech approaches to control Plasmodium parasite transmission by anopheles mosquitoes. Journal of Tropical Medicine 2011: 891342 44 George Edward Barringer III 50 Fox Run Groton,MA 01450 g.edwardbarringer@gmail.com Summary of Qualifications Masters of Science in Molecular Microbiology and Immunology Obtained Industry Experience through Groton Biosystems Quality Control Department Proficient in Plasmodium falciparum culture handling, maintaining mosquito colonies Extensive experience in culturing bacteria, yeast, and halophilic archaea Education Master’s of Science Molecular Microbiology and Immunology. Johns Hopkins School of Public Health GPA: 3.71/4.00 May 2014 Bachelor of Science Biology,Minor in History Ursinus College GPA:3.71/4.00 May 2012 Skills Analytical Technology: fraction collectors, cell counters, biochemistry analyzers, and Automated Reactor Sampling systems Techniques: Trizol & Chloramphenicol extraction, PCR, Quantitative Real time PCR, microscopy, florescent microscopy, UVVIS spectroscopy, nano-injection, maintenance of transgenic mosquito colonies, sterile dissection Culturing: fastidious and non-fastidious bacteria, yeast, extremophile archaea Academic Experience University Bloomberg School of Public Health, Prof. George Dimopoulos Lab Researched inhibition of Plasmodium falciparum in Anopheles gambiae by a Candida sp. Isolated a novel Candida strain from the midgut of An. gambiae Utilized nano-injection of dsRNA to investigate knockdown of mosquito immune pathway components Studied bacteria and yeast interactions in the midgut with ex vivo and in vitro techniques Determined Plasmodium inhibition occurs in the pre-oocyst stage Determined the active anti-Plasmodium factor is secreted, increases bacteria load in the mosquito midgut Ursinus College, Prof. Anthony Lobo Lab, Summer Fellows and Directed Research Johns Hopkins 2012-2014 2011-2012 Researched the molecular mechanism of resistance to sodium selenate of the halophile Haloferax mediterranei Learned culturing techniques for fastidious organisms, Produced and maintained a pure mutant strain of H. mediterranei through increasing exposures to sodium selenate Developed biochemical tests to determine metabolic pathway activation Investigated the nature of precipitate following H. mediterranei exposure to sodium selenate Groton Biosystems, Quality Control Department Technician Summer 2009, 2010 Developed new methodologies for processing samples, filtration test methodology Developed protocols to use viscous samples in the Groton Biosystems ARS reactor sampling system Produced growth media and reagents for experiments 45