File - Science with Ms. Dobrin
advertisement

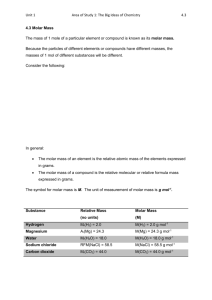
Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms **Calculating Ar and Percent Abundance Example 1: Boron has two naturally occurring isotopes. 10B has a percent abundance of 19.9%, while 11B has a percent abundance of 80.1%. Find the relative atomic mass (Ar). Step 1: Convert percents to decimals 10 B= 0.199 11 B= 0.801 Step 2: Multiply by respective masses 10*0.199= 1.99 11*0.801=8.811 Step 3: Add together 1.99+8.81=10.8 Step 4: Ar is equal 10.8amu (atomic mass units) Example 2: Rubidium has a relative atomic mass of 85.47 and consists of two naturally occurring isotopes, 85Rb (u=84.91) and 87RB (u=86.91). Calculate the percent composition of these isotopes in a naturally occurring sample of rubidium. Step 1: If Ar = 85Rb+87Rb, establish a formula as follows: Ar= 85.47= (84.91x + 86.91(100-x))/100 Step 2: Expand your equation Ar= 8547= 84.91x + 8691-86.91x Step 3: Solve by making x the subject of the expression: Ar= (8547-8691) = -144 = 84.91x-86.91x Ar= -144= -2x Ar= 72 Step 3: 85Rb has a percent abundance of 72.00% and 87Rb has a percent abundance of 28% Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms Calculating Mr and Deducing Empirical and Molecular Formulas Example 3: Calculate the Mr of sulfuric acid, H2SO4 Step 1: Find Ar for each element Hydrogen: 1.01 Sulfur: 32.07 Oxygen: 16.00 Step 2: Multiply by the number of atoms to find the combined mass for each element Hydrogen: 1.01*2= 2.02g Sulfur: 32.07*1= 32.07g Oxygen: 16.00*4= 64.00g Step 3: Add together values for each element 2.02g+32.07g+64.00g= 98.09g mol-1 Example 4: Calculate the percent by mass of sulfur in sulfuric acid, H2SO4 Step 1: Find Mr of sulfuric acid and Ar of sulfur Mr= 98.09g mol-1 Ar= 32.07g mol-1 Step 2: Divide Ar by Mr 32.07/ 98.09 = 0.3269 Step 3: Multiply by 100 to get percent 0.3269*100=32.69% Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms Example 5: Deduce the empirical formula of an organic compound that contains 75% carbon and 25% hydrogen by mass. Step 1: Find the Ar of each element C: 12.01 H:1.01 Step 2: Divide percent composition by Ar C: 75/12.01= 6.24 H: 25/1.01= 24.75 Step 3: Take the smallest quotient (in this case 6.24) and divide both numbers by this value to provide a whole number ratio C: 6.24/6.24= 1 H: 24.75/6.24= 3.97 Step 4: In this case because percent composition is experimentally determined, it is acceptable to round when a value is this close. The difference can be assumed to be experimental error. C: 1 H: 4 Step 5: Use whole number ratio from step 4 as subscripts in determining empirical formula CH4 Example 6: An acid with an empirical formula of HSO4 is found to have a molar mass of 194.13 g mol-1. Deduce the molecular formula. Step 1: Find the empirical formula mass H: 1.01*1= 1.01 S: 32.07*1= 32.07 O: 16.00*4= 64.00 1.01+32.07+64.00= 97.08 g mol-1 Step 2: Divide molar mass by empirical formula mass 194.13g mol-1/ 97.08g mol-1= 1.999=2 Step 3: Multiply each subscript in the empirical formula by the value found in step 2 2(HSO4)= H2S2O8 ** **Review from topic 2, related materials** Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms Example 7: Find the number of atoms of each element in a 150g sample of iron (iii) chloride (FeCl3). Step 1: Find Mr of FeCl3 Fe: 55.85 g mol-1 + Cl: 35.45g mol-1 * 3 = 162.2 g mol-1 Step 2: Use “magic triangle” to find number of moles 150g/162.2g mol-1= 0.93 moles Step 3: Multiply number of moles by Avogadro’s constant 0.93 moles * 6.02x1023= 5.57x1023 molecules of FeCl3 Step 4: In each molecule of iron (iii) chloride, there is 1 iron atom and 3 chlorine atoms, so multiply by these numbers. Fe: 5.57x1023 x 1= 5.57x1023 atoms of Fe Cl: 5.57x1023 x 3= 1.67x1024 atoms of Cl Example 8: In the balanced equation Fe2O3 (s) + 3CO (g) 2Fe (l) + 3CO2 (g), calculate the minimum amount of iron (iii) oxide needed to produce 800g iron. Step 1: Find Mr for the products and reactants Fe2O3= 159.7g mol-1 CO=28.01 g mol-1 Fe=55.85g mol-1 CO2= 44.01g mol-1 Step 2: Use “magic triangle” to find moles by dividing 800g by the molar mass of Fe 800g/ 55.85g mol-1= 14.3240824 moles Step 3: Look at molar ratio to determine moles of iron (iii) oxide needed Molar ratio is 1:2, so in this equation, divide moles of Fe by 2= 7.16204118 moles Fe2O3 Step 4: Use “magic triangle” to mass by multiplying moles by Mr of Fe2O3 7.16204118 moles x 159.7 g mol-1= 1143.77798g or simplified, 1144g of Fe2O3 Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms Example 9: Phosphoric acid is manufactured when molten elemental phosphorous is oxidized and then hydrated. In the following balanced equation, 24.77g of phosphorous reacts with 100g of oxygen and excess water. Determine which the limiting reagent is, and also what mass of phosphoric acid will be produced. P4 (l) +5O2 (g) + 6H2O (l) 4H3PO4 (aq) Step 1: Find the molar mass of phosphorous and oxygen P=30.97x4= 123.88 g mol-1 O= 16.00x2 = 32.00g mol-1 Step 2: Use magic triangle to find moles of each reactant P: 24.77g/123.88g mol-1= 0.19995157moles O: 100g/ 32.00g mol-1= 3.125moles Simplified: P= 0.200moles O= 3.125 moles Step 3: You can ignore H2O because it is stated that the reaction occurs in excess water Step 4: Cross multiply using mole ratios to determine limiting reagent P4: O2 1 :5 0.200: α α equals number of moles of oxygen needed to react with 0.200 moles P4 5x 0.200= 1 1 mole of O2 is needed to react with 0.200 moles of P4 P4 is the limiting reagent, as O2 is in excess Step 5: Use the mole ratio between P4 and H3PO4 to determine moles of H3PO4 produced P4: H3PO4 1 :4 0.200: α α= 0.800 moles Step 6: Convert moles to grams using magic triangle 0.800 moles x 98.00 g mol-1= 78.40g of H3PO4 Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms Example 10: Aspirin is produced when ethanoic anhydride (C4H6O3) is reacted with 2-hydrobenzoic acid, C7H6O3 as seen in the balanced reaction below. 13.80g of 2-hydrobenzoic acid racts with 10.26g ethanoic anhydride and 10.90g aspirin is produced. Determine the percent yield. 2C7H6O3+ C4H6O3 2C9H8O4+ H2O Step 1: Find molar mass of each reactant and product C4H6O3: (12.01x4)+(1.01x6)+(16.00x3)=102.1g mol-1 C7H6O3: (12.01x7)+(1.01x6)+(16.00x3)= 138.13 g mol-1 C9H8O4: (12.01x 9)+(1.01x8)+(16.00x4)=180.17g mol-1 Step 2: Use magic triangle to find moles of each reactant C4H6O3: 10.26g/ 102.1g mol-1= 0.99905886 moles (0.100moles) C7H6O3: 13.80g/ 138.13g mol-1= 0.99978281 moles (0.1000 moles) Step 3: Cross multiple to determine which is the limiting reactant C4H6O3: C7H6O3 1:2 0.1000: α 0.2000 C7H6O3 would be needed to react with 0.1000 moles C4H6O3. C7H6O3 is the limiting reagent Step 4: Determine theoretical yield using mole ratios C7H6O3: C9H8O4 2:2 0.1000: 0.1000 moles C9H8O4 0.1000moles x 180.17g mol-1= 18.017g C9H3O4 Step 5: Divide actual yield be theoretical yiel, then multiply by 100 to get percent yield (10.90g/ 18.017g)x 100= 60.50% yield Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms PRACTICE PROBLEMS: 1. Nitrogen has an Ar of 14.0067 amu. It has two isotopes- 14N and 15N, which have masses of 14.0031 and 15.0001, respectively. What is the percent abundance of each isotope? 2. Lithium has two stable isotopes- 6Li and 7Li. 7Li is by far the most abundant, with a percent abundance of 92.5%, while the remainder is 6Li. What is the Ar of lithium? 3. Cyanogen is a toxic gas consisting of 53.8% nitrogen and 46.2% carbon by mass. What is its empirical formula? 4. If the compound from question 3 is found to have a Mr of 52.04g mol-1, what is its molecular formula? Chemistry SL/HL Relative Atomic Mass, Molar Mass, and Number of Atoms 5. A sample of methanol (CH3OH) is what percent oxygen by mass? 6. 0.1 moles of BaCl2 would have what mass? 7. In the sample in problem 6, how many chlorine atoms are present? 8. In the combustion of propane (shown below), calculate the mass of CO2 produced when 100g of propane is burned. C3H8 (g) + 5)2 (g)= 3CO2 (g) + 4H2O (l) 9. In the combustion of butane (shown below), 8.72g of butane reacts with 28.8g oxygen. Identify the limiting reagent. 2C4H10 (g) + 13O2 (g) 8CO2 (g) + 10H2O (l) Solutions: 1. 14N= 99.63% 2. Li= 6.925amu 3. CN 4. (CN)2 or C2N2 5. 49.9% oxygen 6. 20.82g 15 N= 00.37% Chemistry SL/HL 7. 1.2x1023 atoms of chlorine 8. 299g 9. C4H10 is in excess Relative Atomic Mass, Molar Mass, and Number of Atoms