PERCENTAGE COMPOSITION AND EMPIRICAL FORMULA The
advertisement

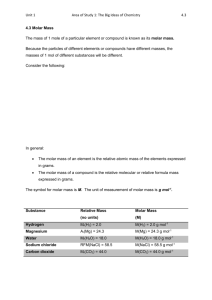
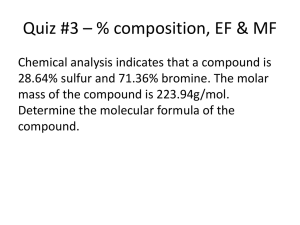
PERCENTAGE COMPOSITION AND EMPIRICAL FORMULA The Mole concept: As atoms and molecules are very small, one gram of any substance would contain millions of atoms or molecules. Common units used in everyday life like the dozen are too small and impractical to use when counting atoms. To overcome this difficulty a unit called the " mole" is used. As the mass of the carbon-12 isotope has been accurately determined, the definition of the mole has been based on this isotope. The mole is the number which is equivalent to the number of atoms in 12 g of carbon-12. This number is called Avogadro's Number, NA, and is equal to 6,02 x 1023. As one mole of carbon-12 atoms has a mass of 12 g, it follows that the mass of one mole of any element will be equal to the atomic number in grams, for example, one mole of chlorine atoms has a mass of 35,5 g. It has been found that one mole of any gas occupies a volume of 22,4 dm3 at standard temperature and pressure (S.T.P.). This is known as the molar gas volume. Molar Mass or formula mass: The molar mass of an element or compound is equal to the mass in grams of one mole of atoms or molecules. The molar mass of a compound is often referred to as its formula mass. To determine the molar mass of a compound, the atomic masses of the individual atoms are added. The unit of molar mass is the g.mol-1. Examples: Molar mass of CO2 = 12 + 2(16) = 44 g.mol-1 Molar mass of Cu(NO3)2 = 63,5 + 2(14 + 48) = 187,5 g.mol-1 Percentage Composition The percentage composition of a compound gives the proportion by mass in which constituent elements are present in compounds as percentages. Molar masses are used in calculating percentage compositions. Examples: (1) Calculate the percentage composition of sodium sulphate. Formula: Na2SO4 M(Na2SO4) = 2(23) + 32 + 4(16) = 142 g.mol-1 Na: S: O: (2) 46 100 32,39 % 142 32 100 22,54 % 142 64 100 45,07 % 142 Calculate the percentage of water of crystallisation in CuSO4.5H2O. M(CuSO4.5H2O) = 63,5 + 32 + 4(16) + 5(18) = 249,5 g.mol-1 90 100 36,07 % H2O: 249,5 Empirical Formula The simplest formula or empirical formula of a compound indicates the ratio in which elements occur in a compound. This does not necessarily give the actual molecular formula, as the molecular formula may be a multiple of the simplest formula. The simplest formula is determined from the percentage composition of the unknown compound. Examples: 1. A compound contains 31,89% potassium, 28,94% chlorine and 39,17% oxygen. Determine its simplest formula. K Cl O Determine the mole fraction by dividing the percentage by the molar mass. 31,89 39 28,94 35,5 39,17 16 Divide by the smallest number to bring the ratio to whole numbers. 0,82 0,82 0,82 0,82 2,45 0,82 1 3 1 Simplest formula: KClO3. 2. 62,94% of Na2CO3.xH2O consists of water of crystallisation. Calculate the number of molecules of water. M(Na2CO3) = 2(23) + 12 + 3(16) = 106 g.mol-1 M(H2O) = 2(1) + 16 = 18 g.mol-1 % Na2CO3 = 100 - 62,94 = 37,06% Na2CO3 H2O 37,06 106 62,94 18 0,35 0,35 3,5 0,35 1 10 Simplest formula: Na2CO3.10H2O There are 10 molecules of water of crystallisation. 3. An organic compound has the following percentage composition; 65,01% carbon, 8,76% hydrogen and 26,23% oxygen. The molar mass of the compound was found to be 368 g.mol-1. Determine the molecular formula. Determine the simplest formula: C H O 65,01 12 8,76 1 26,23 16 5,42 1,64 8,76 1,64 1,64 1,64 3,30 10 5,34 1 16 3 (Convert to approximate whole numbers by multiplying by three.) Simplest formula: C10H16O3 Determine the molecular formula: M(C10H16O3) = 10(12) + 16(1) + 3(16) = 184 g.mol-1 Actual M = 386 g.mol-1. Molecular formula = 2 x simplest formula = C20H32O6.