Brazill exam questions
advertisement
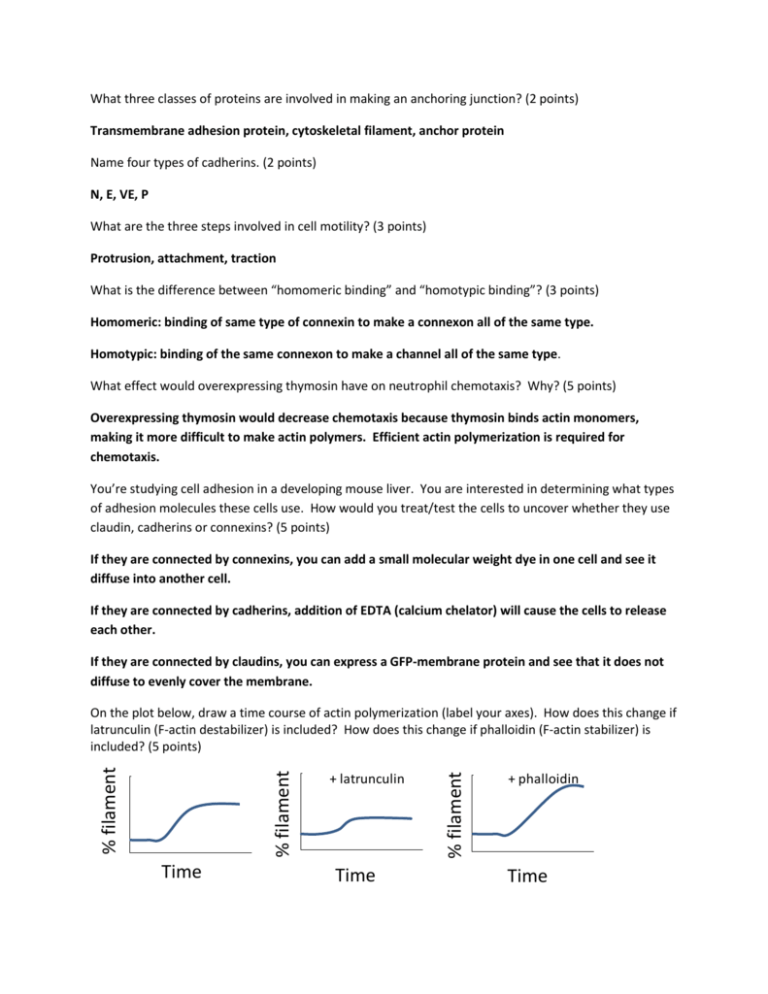
What three classes of proteins are involved in making an anchoring junction? (2 points) Transmembrane adhesion protein, cytoskeletal filament, anchor protein Name four types of cadherins. (2 points) N, E, VE, P What are the three steps involved in cell motility? (3 points) Protrusion, attachment, traction What is the difference between “homomeric binding” and “homotypic binding”? (3 points) Homomeric: binding of same type of connexin to make a connexon all of the same type. Homotypic: binding of the same connexon to make a channel all of the same type. What effect would overexpressing thymosin have on neutrophil chemotaxis? Why? (5 points) Overexpressing thymosin would decrease chemotaxis because thymosin binds actin monomers, making it more difficult to make actin polymers. Efficient actin polymerization is required for chemotaxis. You’re studying cell adhesion in a developing mouse liver. You are interested in determining what types of adhesion molecules these cells use. How would you treat/test the cells to uncover whether they use claudin, cadherins or connexins? (5 points) If they are connected by connexins, you can add a small molecular weight dye in one cell and see it diffuse into another cell. If they are connected by cadherins, addition of EDTA (calcium chelator) will cause the cells to release each other. If they are connected by claudins, you can express a GFP-membrane protein and see that it does not diffuse to evenly cover the membrane. Time + latrunculin Time % filament % filament % filament On the plot below, draw a time course of actin polymerization (label your axes). How does this change if latrunculin (F-actin destabilizer) is included? How does this change if phalloidin (F-actin stabilizer) is included? (5 points) + phalloidin Time You are studying cAMP chemotaxis in Dictyostelium discoideum. You have uncovered a mutant that cannot chemotax towards cAMP. How would you determine whether this defect is a general defect in chemotaxis or a defect specific to cAMP chemotaxis? You find that it is specific to cAMP chemotaxis. Your lab mate who performed the chemotaxis assays informs you that the mutants have normal speed but decreased directionality. What does this mean? Given this, you decide to perform a cAMPstimulated F-actin polymerization assay. Here are your results: W T F - a c t in ( r e la t iv e t o W T t = 0 ) 2 m u ta n t 1 0 20 40 60 80 100 120 140 160 T im e ( s e c ) What does this tell you about F-actin polymerization in response to cAMP? Provide a hypothesis that biochemically explains the mutant’s defective behavior. Describe an experiment to test this hypothesis. (15 points) Perform a chemotaxis assay using vegetative cells and folate. If it is a general chemotaxis defect, then the cells will not chemotax well to folate. If it is specific to cAMP chemotaxis, then chemotaxis to folate will be normal. This means that the cells have normal motility, but are unable to move up the cAMP gradient efficiently. F-actin polymerization in response to cAMP is defective in the mutant. The mutant is defective in sensing cAMP. It may have a defective cAR1 (cAMP receptor) that cannot bind cAMP. To test this hypothesis, I will perform cAMP binding assays comparing wild type and mutant cells. If the mutant cells don’t bind cAMP as well as the wild type cells, then my hypothesis is supported. If the mutants do bind cAMP as well as the wild type cells, then my hypothesis is incorrect.











