Unit 8 – Solutions and Solubility Test, Version A
advertisement

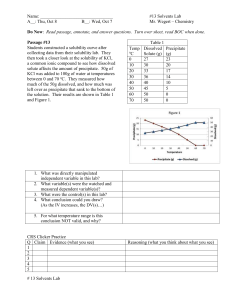
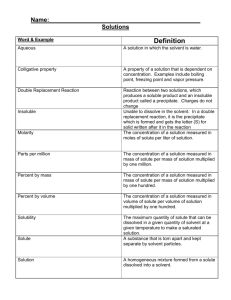
Unit 8 – Solutions and Solubility Test, Version A 1. Which of the following Lewis structures best represents the geometry of a water molecule? B. C. D. A. 2. Unlike most other substances, water becomes denser when it melts because A. water molecules increase in mass when ice melts to water. B. water molecules in ice are in a random arrangement that changes to a tight geometric arrangement when ice melts. C. the arrangement of water molecules in water is more ordered than the arrangement of water molecules in ice is. D. water molecules in ice are arranged in an open pattern that collapses when ice melts. 3. Why doesn’t water in lakes and ponds of temperate climates freeze solid during the winter and kill nearly all the living things it contains? A. water has a cooling effect B. ice floats and insulates the water underneath C. the air above the water keeps it warm D. salt water contains too much salt to allow freezing 4. Crystals of ammonium chloride, NH4Cl, seem to disappear when mixed with water. The resulting clear liquid containing ammonium chloride and water is a A. heterogeneous mixture. B. compound. C. homogeneous mixture. D. new substance. 5. Mixtures that exhibit the Tyndall effect are A. alloys. B. solutions. C. colloids. D. homogeneous mixtures. 6. Saltwater is an electrolyte because it contains A. suspended particles B. no solvent. C. water molecules. D. dissociated ions. 7. Substances that are electrolytes A. always conduct an electric current when dissolved in water B. are always composed of molecules. C. always form neutral particles when dissolved in water. D.. are always composed of salts. 8. Which of the following substances is an electrolyte? A. sugar B. potassium chloride, KCl C. water D. salad oil 9. Crushing a solid substance increases the rate at which the substance dissolves because crushing A. causes the particles of the substance to become disarranged. B. increases the surface area of solute that is exposed to solvent. C. decreases the concentration of the substance. D. increases the kinetic energy of the particles of the solute. 10. Stirring or shaking a mixture of solid solute and solvent increases the rate of dissolving by A. increasing the kinetic energy within the mixture. B. increasing the surface area of the undissolved solute to the solvent. C. allowing air bubbles to move the solvent more vigorously. D. constantly bringing fresh solvent into contact with undissolved solute. 11. Which of these is true about alloys? A. alloys are homogeneous mixtures of two or more metals that combine their properties B. alloys are polar compounds of metals that demonstrate the “like dissolves like” property C. alloys produce the Tyndall effect when mixed with water and lit with a flashlight D. alloys are usually more expensive and more precious than the individual metals 12. Consider the possibility of each of the following substances dissolving in water. Which of these substances best demonstrates the principle "like dissolves like"? A. potassium bromide B. sulfur C. olive oil D. oxygen gas 13. As temperature increases, the solubility of gases A. increases. B. decreases. C. stays the same. D. is not affected. 14. A dilute solution is a solution that A. contains a small amount of dissolved solute. B. contains a small amount of dissolved solvent. C. is concentrated. D. is saturated. 15. A certain solution contains 38.75 g of nickel (II) iodide, NiI2, in 1.50 L of solution. What is the molarity of the solution? A. 25.8 M B. 0.0827 M C. 0.124 M D. 0.0888 M 16. Jonathan is reading the solubility chart and finds that KNO3 is soluble. What symbol would he use in his chemical equation to show this? A. (s) B. (l) C. (aq) D. (g) 17. What mass of NaOH is dissolved in 25.00 mL of a 4.500 M solution of NaOH? A. 4.444 g NaOH B. 0.1125 g NaOH C. 0.2222 g NaOH D. 4.500 g NaOH 18. If you were making a 0.500M solution of hydrochloric acid in a 3.00L bottle, how many liters of 6.00M HCl would need to be added? A. 0.300L B. 0.25L C. 1.00L D. 6.00L 19. If 80 mL of an 8.0M acetic acid solution was diluted into a 1.00L container, what would the new concentration be? A. 640 M B. 10.0 M C. 0.64 M D. 0.001 M 20. Which properties below are correctly matched? A. solids are more soluble in liquids at lower pressures and lower temperatures B. solids are more soluble in liquids at higher pressures and lower temperatures C. gases are more soluble in liquids at higher pressures and lower temperatures D. gases are more soluble in liquids at lower pressures and higher temperatures 21. What is the formula for Copper (II) Chloride? A. CuCl B. CuCl2 C. Cu2Cl 22. How many grams are present in 56 moles of copper (II) fluoride? (express your A. 5684 g B. 5700g C. 57g D. 56g 23. According to VSEPR theory the shape of NH3 is _____________________ A. Linear B. Pyramidal C. Tetrahedral D. Triangular planer 24. According to the solubility curves, a temperature change of 30OC to 90 oC has the least effect on the solubility curve of a. KNO3 b. HCl c. NaCl d. NaNO3 25. Which saturated solution has the most amount of solute dissolved in solution? a. KNO3 at 70oC b. KI at 0 oC c. NH3 at 100 oC d. NaNO3 at 80 oC 26. While writing electron configuration we must fill the lower energy orbitals before filling higher energy orbitals is the rule given by A. Hund B. Pauli C. Aufbau 27. Which of the following element is most electronegative: F, Cl, Na, Mg? A. F B. Cl C. Mg D. Na 28. Which of the following is the correct name for the compound Fe2O3? A. Iron Oxide B. Iron (II) Oxide C. Iron (III) oxide 29. Using a periodic table, predict which period has the largest radius? A. 1 B. 3 C. 6 D. 7 30. What is the percentage by mass of Na in a formula unit of NaHCO3 A. 44.2% B. 37.7% C. 27.4% D. 16.7% 31. Which of the following salt is soluble? A. BaSO4 B. CaCO3 C. Fe (OH) 2 D. K3PO4 32. Water molecules cling with each other because of the following property of water A. Cohesion B. Adhesion C. Surface tension D. High specific heat capacity E. Capillary action 33. Water molecules stick with the walls of the container because of the following property of water A. Cohesion B. Adhesion C. Surface tension D. High specific heat capacity E. Capillary action 34. Water rises up the in the shoots when the roots are watered because of the following property of water A. Cohesion B. Adhesion C. Surface tension D. High specific heat capacity E. Capillary action 35. Big cruise ships travel on water because of the following property of water A. Cohesion B. Adhesion C. Surface tension D. High specific heat capacity E. Capillary action 36. On a hot summer day when everything else is hot but water is still cold, is because of the following property of water A. Cohesion B. Adhesion C. Surface tension D. High specific heat capacity E. Capillary action 37. When two liquids completely mix with each other, they are called A. Immiscible B. Miscible C. Dilute D. Concentrated 38. A Solution which can dissolve extra solute with an increase in temperature is called a________________ solution A. Saturated B. Unsaturated C. Supersaturated 39. Which of the following property is intensive A. Mass B. Density C. Volume D. Heat 40. Water is a universal solvent because it is A. Linear B. Polar C. Non Polar D. Has a bond angle of 120. Name Period Unit 11 – Solutions and Solubility Test