CIRB Protocol Deviation Report Form
advertisement

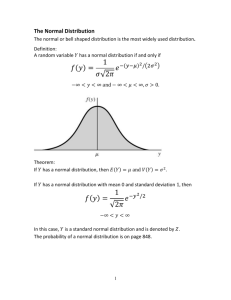
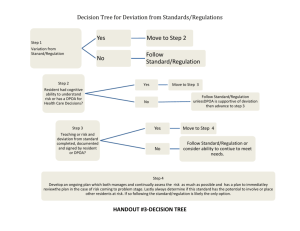
CIRB PROTOCOL DEVIATION/NON-COMPLIANCE REPORT FORM 1. CIRB Reference No: Text Field 2. Protocol Title: Text Field 3. Principal Investigator: Text Field 4. Designation/ Department/ Institution: Text Field 5. Description of Event a. Please describe in detail the nature of the event including the date of occurrence. (If subjects are involved, please indicate the subject identifier.) Text Field b. Please describe why or how the event occurred. Describe the outcome. Text Field c. In your judgement, did the event affect the safety, rights or well-being of the Research Participant (and/or others)? Text Field d. Please describe any corrective action, if applicable, taken for the event. Text Field e. Please describe any preventive action plan to prevent the recurrence of the event in future. Text Field f. Any other comments. Text Field g. Has this event been reported to the Study Sponsor/Grant Body? Not Applicable as there is no study sponsor or grant body. Yes. Please describe response if any. Text Field No. Please provide rationale for not reporting. Text Field CIRB Protocol Deviation/Non-Compliance Report Form Version 2.0, 17 Dec 2013 Page 1 of 2 9. Declaration Of Principal Investigator: I confirm that the information submitted in the above report is true and accurate at the date of submission of the report. ___________________________________ Principal Investigator’s Signature Full Name: Institution: Department: ________________________ Date Text Field Choose from list Text Field Guidance for CIRB Protocol Deviation/Non-Compliance Report Form This report form should be submitted to the CIRB secretariat once PI is aware of the protocol deviation/noncompliance but no more than 14 calendar days. All sections must be completed. PIs are obliged to suspend their research immediately pending their report to the IRB if deviations are substantial or will likely result in greater harm or greater likelihood of harm to the subjects. Definitions Protocol Deviation: is an unplanned excursion from the protocol that is not implemented or intended as a systematic change. A protocol deviation could be a limited prospective exception to the protocol (e.g. agreement between sponsor and investigator to enrol a single subject who does not meet all inclusion/exclusion criteria). Like protocol amendments, deviations initiated by the investigator must be reviewed and approved by the IRB and the sponsor prior to implementation, unless the change is necessary to eliminate an immediate hazard to the human subjects. Protocol deviation is also used to refer to any other, unplanned, instance(s) of protocol noncompliance. For example, situations in which the investigator failed to perform tests required by the protocol or failures on the part of the subjects to complete scheduled visits as required by the protocol. Non-Compliance: is a failure by an investigator or any study team member to abide by the policies and procedures of CIRB or applicable regulations governing the protection of human subject research. Some examples of non-compliance include but are not limited to: Failure to obtain prior approval for research Failure to obtain renewal of approval for research Failure to obtain informed consent when required Failure to use the latest CIRB approved version of the protocol or consent form Failure to report an adverse event report according to the CIRB timeline and procedure Performance of research at an unapproved study site Performance of a drug trial without a valid HSA Clinical Trial Certificate (CTC) Any other failure to adhere to regulations, policies and procedures related to research For CIRB Official Use Only Category of Review Full Board Review Expedited Review Note and File Request for additional Information Follow up action required ________________________ Reviewer’s Name _______________________ ___________________ Reviewer’s Signature Date CIRB Protocol Deviation/Non-Compliance Report Form Version 2.0, 17 Dec 2013 Page 2 of 2