Pre-Audit Checklist
advertisement
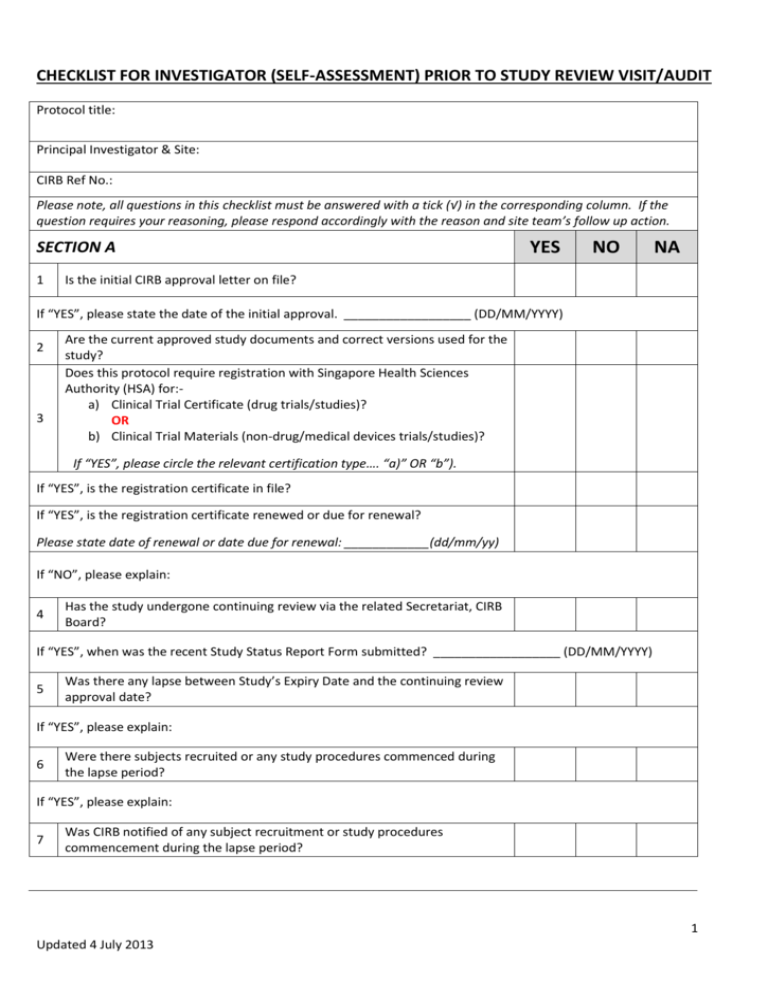
CHECKLIST FOR INVESTIGATOR (SELF-ASSESSMENT) PRIOR TO STUDY REVIEW VISIT/AUDIT Protocol title: Principal Investigator & Site: CIRB Ref No.: Please note, all questions in this checklist must be answered with a tick (√) in the corresponding column. If the question requires your reasoning, please respond accordingly with the reason and site team’s follow up action. SECTION A 1 YES NO NA Is the initial CIRB approval letter on file? If “YES”, please state the date of the initial approval. __________________ (DD/MM/YYYY) 2 3 Are the current approved study documents and correct versions used for the study? Does this protocol require registration with Singapore Health Sciences Authority (HSA) for:a) Clinical Trial Certificate (drug trials/studies)? OR b) Clinical Trial Materials (non-drug/medical devices trials/studies)? If “YES”, please circle the relevant certification type…. “a)” OR “b”). If “YES”, is the registration certificate in file? If “YES”, is the registration certificate renewed or due for renewal? Please state date of renewal or date due for renewal: ____________(dd/mm/yy) If “NO”, please explain: 4 Has the study undergone continuing review via the related Secretariat, CIRB Board? If “YES”, when was the recent Study Status Report Form submitted? __________________ (DD/MM/YYYY) 5 Was there any lapse between Study’s Expiry Date and the continuing review approval date? If “YES”, please explain: 6 Were there subjects recruited or any study procedures commenced during the lapse period? If “YES”, please explain: 7 Was CIRB notified of any subject recruitment or study procedures commencement during the lapse period? 1 Updated 4 July 2013 YES 8 NO NA Has there been any amendments to: PROTOCOL? OR STUDY APPLICATION FORM (FOR STUDIES WITHOUT PROTOCOL)? PATIENT INFORMATION SHEET/INFORMED CONSENT FORM? OTHER ESSENTIAL STUDY RELATED DOCUMENT, example; STUDY RELATED QUESTIONNAIRES? If there were amendments to any of the study documents mentioned above, 9 was the amendment approved by CIRB with the approval letter from CIRB? Were the amended study documents used only after obtaining the CIRB 10 approval letter? If “NO”, please explain: If you answered “NO” to the question above, was CIRB notified? 11 Was the recruitment materials (e.g. posters, flyers, advertisements etc.) used approved by CIRB? 12 Is the study completed? If “YES”, has CIRB been informed? 13 Has there been any premature termination/suspension to the study? If “YES”, please explain: SECTION B 1 Have there been any Serious Adverse Events (SAEs)? If “YES”, please state the number of SAEs and;Were the SAEs reported in a timely manner to CIRB, according to CIRB SAE reporting guidelines? 2 Does the site maintain the Investigational Product (IP)/Device dispensing and accountability log? 3 Is there proper documentation e.g. – temperature log or accessibility log in place for IP/Device storage? 1 Are samples collection methods as per and in compliance to the current approved protocol? If “NO”, please explain: 2 Are the samples coded? 2 Updated 4 July 2013 YES SECTION C 3 If the samples are stored, is the storage area secure with access control to study team members delegated in the Duty Delegation Log? 4 If the samples are stored, is a temperature log maintained? 5 6 NO NA If the samples are shipped, are the records filed in the Study Master File with site? If the samples are to be destroyed after the completion of the study, are the following measures under taken: Is the destruction done as per the institution’s SOP? Will the destruction records be maintained at site in the Study Master File? 7 Is there Case Report Forms (CRF) designed for this study? 8 Is the data captured in the CRF captured in an electronic database system? 9 Are the study personnel who have access to the database identified in the Duty Delegation Log? 10 Is the study database password protected? SECTION D 1 2 Correct Informed Consent Form (ICF) document – was the relevant approved version used? Did the Subject/Legally Acceptable Representative sign and date the ICF personally? 3 Did the person administering the consent, sign and date the ICF personally? 4 Is the person administering the consent assigned in the Duty Delegation Log by Principal Investigator/Site PI? 5 Is there consent process documentation in case notes/medical records? 6 Is a copy of the signed ICF given to subject, and documented in case notes/ medical records? SECTION E 1 Does the study have a filing system for all relevant and essential study documents, e.g. Study Master File? Additional comments/explanations: COMPLETED BY PRINT NAME: SIGNATURE: 3 Updated 4 July 2013 ROLE IN STUDY: DATE: (dd/mm/yyyy) 4 Updated 4 July 2013