Graphs - Uplift North Hills
advertisement
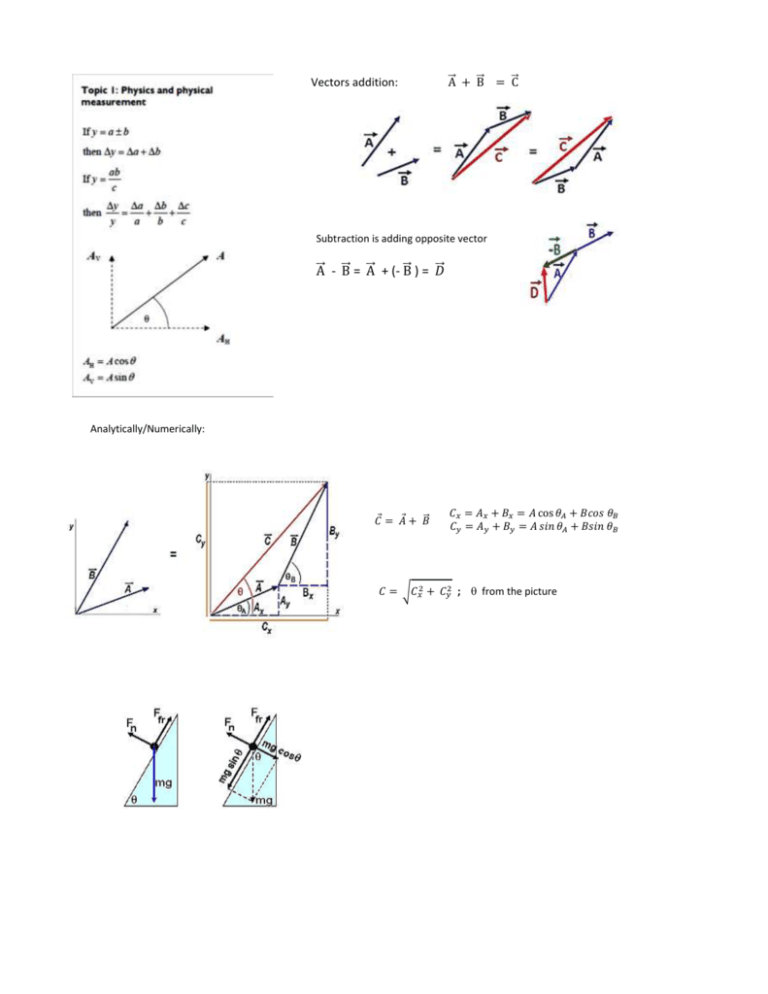
Vectors addition: ⃗A + ⃗B = ⃗C Subtraction is adding opposite vector ⃗ - B ⃗ + (- B ⃗ = A ⃗ )= 𝐷 ⃗ A Analytically/Numerically: ⃗ 𝐶 = 𝐴+ 𝐵 𝐶𝑥 = 𝐴𝑥 + 𝐵𝑥 = 𝐴 cos 𝜃𝐴 + 𝐵𝑐𝑜𝑠 𝜃𝐵 𝐶𝑦 = 𝐴𝑦 + 𝐵𝑦 = 𝐴 𝑠𝑖𝑛 𝜃𝐴 + 𝐵𝑠𝑖𝑛 𝜃𝐵 𝐶 = √𝐶𝑥2 + 𝐶𝑦2 ; from the picture Displacement (a vector quantity) is a measured distance in a given direction (m). Graphs: Average velocity: slope of the straight line joining the initial and final position on the position time graph. (Instantaneous) velocity at a given point: slope of the tangent line at given time on the position time graph. Average Acceleration: slope of the straight line joining the initial and final position on the velocity - time graph. (Instantaneous) acceleration at a given point: slope of the tangent line at given time on the velocity - time graph Displacement is the area under velocity – time graph Change in velocity is the area under acceleration – time graph Air resistance provides a drag force to objects in free fall. ▪ The drag force increases as the speed of the falling object increases resulting in decreasing downward acceleration ▪ When the drag force reaches the magnitude of the gravitational force, the falling object will stop accelerating and fall at a constant velocity. This is called the terminal velocity/speed. Inertia is resistance an object has to a change of velocity Mass is numerical measure of the inertia of a body (kg) Weight is the gravitational force acting on an object . W = mg Force is an influence on an object that causes the object to accelerate. • 1 N is the force that causes a 1-kg object to accelerate 1 m/s2. Fnet (resultant force) is the vector sum of all forces acting on an object Free Body Diagram is a sketch of a body and all forces acting on it. Newton’s first law: An object continues in motion with constant speed in a straight line (constant velocity) or stays at rest unless acted upon by a net external force. Object is in translational equilibrium if Fnet = 0, a = 0 no change in velocity Δp is the change in momentum produced Newton’s second law:: F = Δp Δt by the net force F in time Δt. Δp = Δ(mv) two simple cases: 1. velocity changes, mass doesn’t change: Δp = mΔv → F = ma old form of Newton’s second law: If a net force is acting on an object of mass m, object will acquire acceleration Direction of acceleration is direction of the net force. 2. mass changes, velocity doesn’t change: Δp = vΔm → F = ∆m v ∆t . Δm/Δt in (kg/s) Newton’s third law: Whenever object A exerts force on object B, object B exerts an equal in magnitude but opposite in direction force on object A Normal/Reaction force (Fn or R) is the force which is preventing an object from falling through the surface of another body . That’s why normal force is always perpendicular (normal) to the surfaces in contact. Friction force is the force that opposes slipping (relative motion ) between two surfaces in contact; it acts parallel to surface in direction opposed to slipping. Friction depends on type and roughness of surfaces and normal force. Linear momentum is defined as the product of an object’s mass and its velocity: p = mv vector! (kg m/s) Impulse is defined as the product of the net force acting on an object and time interval of action: FΔt vector! (Ns) Impulse F∆t acting on an object will produce the change of its momentum Δp: F∆t = ∆p Δp = mv - mu Ns = kg m/s Achieving the same change in momentum over a longer time requires smaller force, and over a shorter time requires greater force. WHEN YOU TRY TO FIND CHANGE IN MOMENTUM REMEMER TO LABEL VELOCITIES AS POSITIVE OR NEGATIVE The impulse of a time-varying force is represented by the area under the force-time graph. Law of Conservation of Momentum: The total momentum of a system of interacting particles is conserved - remains constant, provided there is no resultant external force. Such a system is called an “isolated system”. momentum of the system after collision = momentum of the system before collision (p = pi ) vector sum REMEMBER TO DRAW A SKETCH OF THE MASSES AND VELOCITIES BEFORE AND AFTER COLLISION. LABEL VELOCITIES AS POSITIVE OR NEGATIVE. Elastic collision: both momentum and kinetic/mechanical energy are conserved. That means no energy is converted into thermal energy Inelastic collision: momentum is conserved but KE is not conserved. Perfectly inelastic collision: the most of KE is converted into other forms of energy when objects after collision stick together. To find how much of KE is lost in the system, subtract KE of the system after collision from KE of the system before the collision. If explosion happens in an isolated system momentum is conserved but KE increases (input of energy from a fuel or explosive material.) . Work is the product of the component of the force in the direction of displacement and the magnitude of the displacement. (scalar) W = Fd cos Ѳ (Fd = F cos Ѳ) (Joules) Work done by a varying force F along the whole distance travelled is the area under the graph FcosѲ versus distance travelled. the work done by centripetal force is zero: Wnet = 0 → Wnet = ∆KE → no change in KE → no change in speed; centripetal force cannot change the speed, only direction. Examples: gravitational force on the moon, magnetic force on the moving charge. Energy is the ability to do work. Work changes energy. Potential energy is stored energy. (Change in) Gravitational potential energy ∆EP = mg ∆h Elastic potential energy = work is done by external force in stretching/compressing the spring by extension x. EPE = W = ½ kx2 Kinetic energy is the energy an object possesses due to motion EK = ½ mv2 Work done byapplied/external force changes potential energy (when net force is zero, so there is no acceleration). Work done by net force changes kinetic energy (net force gives acceleration, therefore changes velocity). Work – Kinetic energy relationship: work done by net force changes kinetic energy: W = ∆KE = final EK – initial EK = ½ mv2 – ½ mu2 Conservation of energy law: Energy cannot be created or destroyed; it can only be changed from one form to another. For the system that has only mechanical energy (ME = potential energies + kinetic energy) and there is no frictional force acting on it, so no mechanical energy is converted into thermal energy, mechanical energy is conserved ME1 = ME2 = ME3 = ME4 mgh1 + ½ mv12 = mgh2 + ½ mv22 = • • • • • • If friction cannot be neglected we have to take into account work done by friction force which doesn’t belong to the object alone but is shared with environment as thermal energy. Friction converts part of kinetic energy of the object into thermal energy. Frictional force has dissipated energy: ME1 – Ffr d = ME2 (Wfr = – Ffr d) Power is the rate at which work is performed or the rate at which energy is transmitted/converted. scalar (1 W(Watt) = 1 J/ 1s ) Another way to calculate power P = Fv W E P out out out Efficiency is the ratio of how much work, energy or power we get out of a system compared to how much is put in. eff = W = E = P in in in Centripetal acceleration causes change in direction of velocity, but doesn’t change speed. It is always directed toward the center of the circle. ac = v2 r Centripetal force: Fc = mac = Period T: mv 2 r time required for one complete revolution/circle speed around circle of radius r: v= 2πR T Macroscopic level: temperature gives indication of the degree of hotness or coldness of a body, measured by thermometer. Thermal equilibrium occurs when all parts of the system are at the same temperature. There is no exchange of thermal energy/please do not mention heat. (This is how a thermometer works) Thermal energy of a system = internal energy—the sum total of the potential energy and kinetic energy of the particles making up the system. Potential energy of the molecules arises from the forces (bonds/ because of intermolecular forces) between them. Kinetic energy of the molecules arises from the translational, rotational, ad vibrational motion of the particles. Microscopic level: (absolute) temperature directly proportional to the average kinetic energy of the molecules of a substance: (KE)avg = 3⁄2 kT. k is Boltzmann constant Heat is the thermal energy that flows/is transferred from one body or system of higher temperature to another of lower temperature. Relative atomic mass is the mass of an atom in units of 1/12 of the mass of a carbon-12 atom. The mole is the amount of substance that contains the same number of atoms/molecules as 0.012 kg of carbon-12. Molar Mass is the mass of one mole of a substance (kg/mol). 1 mole of a gas at STP occupies 22.4 l (dm3) and contains 6.02 x 1023 molecules/mol. Heat/Thermal Capacity is the amount of thermal energy needed to raise the temperature of a substance/object by one degree Kelvin. C = Q ∆T → Q = C ΔT unit: (C) = J K-1 Specific heat capacity is the quantity of thermal energy required to raise the temperature of one kilogram of a substance by one degree Kelvin. 𝑄 c = → Q = mc ΔT unit: (c) = J kg-1 K-1 homogeneous substance: C = mc 𝑚∆𝑇 amount of thermal energy needed to increase the temperature of m kg of a substance with specific heat capacity c by ΔT amount of thermal energy released when the temperature of m kg of a substance with specific heat capacity c decreases by ΔT Latent heat is the thermal energy that a substance/body absorbs or releases during a phase change at constant temperature. Qat const. temp. unit: J Specific latent heat is the thermal energy required for a unit mass of a substance to undergo a phase change. Q L= → Q = mL unit: (L) = J/kg m If electrical energy is converted into increase of internal energy of the system, then: 4 Phases (States) of Matter Characteristic Qadded = electrical energy = Pt = IVt = Qabosorbed P – power, I – current, V – voltage, t - time solid, liquid, gas and plasma; ordinary matter – only three phases Solid Volume and shape definite volume and definite shape Compressibility Almost Incompressible Bonds = intermolecular forces characterized by high density and the molecules are held in fixed position by strong bonds. Molecules vibrate around a mean (equilibrium) position. Comparative Density High Kinetic Energy Vibrational Potential Energy Mean molecular Separation High r0 ( size of the particle) Liquid Gas definite volume but its shape can change – it takes the shape of their containers. Very Slightly Compressible density is lower and molecules are further apart without fixed positions Molecules experience little resistance to motion and move freely about. There are still strong forces between the molecules but they are free to move around each other. High Vibrational, rotational, some translational Higher neither definite volume nor definite shape Highly Compressible > r0 Phase transition is the transformation of a thermodynamic system from one phase to another. Changes: S → L melting or fusion S → G sublimation L → G vaporization includes boiling and evaporation L → S freezing or solidification G → S deposition or desublimation G → L condensation the forces between molecules are very weak – molecules are essentially independent of one another but they do occasionally collide Low Mostly translational, higher rotational and vibrational Highest (ideal gas – zero) 10 - 100 r0 While melting, vibrational kinetic energy increases and particles gain enough thermal energy to break from fixed positions. Potential energy of system increases. Melting point of a solid is the temperature at which it changes state from solid to liquid. Once at the melting point, any additional heat supplied does not increase the temperature. Instead is used to overcome the forces between the solid molecules increasing potential energy. ◌ At the melting point the solid and liquid phase exist in equilibrium. While freezing, particles lose potential energy until thermal energy of the system is unable support distance between particles and is overcome by the attraction force between them. Kinetic energy changes form from vibrational, rotational and part translational to merely vibrational. Potential energy decreases (It is negative!!! – attraction – intermolecular forces become stronger). to While boiling, substance gains enough potential energy to break free from inter-particle forces. Similar to evaporation, the only difference being that energy is supplied from external source so there is no decrease in temperature. While condensing, the energy changes are opposite to that of boiling. The distinguishing characteristic of a phase transition is an abrupt change in one or more physical properties, in particular the heat capacity, and the strength of intermolecular forces. During a phase change, the thermal energy added or released is used to change (increase/decrease) the potential energy of the particles to either overcome or succumb to the inter-molecular force that pulls particles together. In the process, the average kinetic energy will not change, so temperature will not change. Evaporation is a change of phase from the liquid state to the gaseous state that occurs at a temperature below the boiling point. Evaporation causes cooling. A liquid at a particular temperature has a range of particle energies, so at any instant, a small fraction of the particles will have KE considerably greater than the average value. If these particles are near the surface of the liquid, they will have enough KE to overcome the attractive forces of the neighbouring particles and escape from the liquid as a gas. The escape of the higher-energy particles will lower the average kinetic energy and thus lower the temperature. The rate of evaporation is the number of molecules escaping the liquid per second. Evaporation can be increased by • increasing temperature/more particles have a higher KE • Increasing surface area/more particles closer to the surface • Increasing air flow above the surface (gives the particles somewhere to go to)/ decreasing the pressure of the air above liquid Kinetic Model of an Ideal Gas help: PV = NkT P – pressure, V – volume, N number of particles, k – Boltzmann constant, T - temperature Gas pressure is the force gas molecules exert due to their collisions (with a wall – imaginary or real). P= 𝐹 A (pressure = force/area) Assumptions of the kinetic model of an ideal gas. • Gases consist of tiny hard spheres/particles called atoms or molecules. • The total number of molecules in any sample of a gas is extremely large. • The molecules are in constant random motion. • The range of the intermolecular forces is small compared to the average separation of the molecules. • The size of the particles is relatively small compared with the distance between them. • No forces act between particles except when they collide, and hence particles move in straight lines. • Between collisions the molecules obey Newton’s Laws of motion. • Collisions of short duration occur between molecules and the walls of the container and the collisions are perfectly elastic (no loss of kinetic energy). Temperature is a measure of the average random kinetic energy of the molecules of an ideal gas. Macroscopic behavior of an ideal gas in terms of a molecular model. • Increase in temperature is equivalent of an increase in average kinetic energy (greater average speed). This leads to more collisions and collisions with greater impulse. Thus resulting in higher pressure. • Decrease in volume results in a smaller space for gas particles to move, and thus a greater frequency of collisions. This results in an increase in pressure. Also, depending on the speed at which the volume decreases, particles colliding with the moving container wall may bounce back at greater speeds. This would lead to an increase in average kinetic energy and thus an increase in temperature. • An increase in volume would have an opposite effect. Simple Harmonic Motion is periodic motion in which the acceleration/ restoring force is proportional to and in opposite direction of the displacement. a = − ω2x. spring: a = F/m = -kx/ m = -(k/m) x = = − ω2x ω2 = k/m General equation for the position of a particle undergoing simple harmonic motion: x = x0 cos ωt. x0 – amplitude, f – frequency f = 1/T T – period for full oscillation angular frequency = 2 f (how many full circles 2 per second) = 2/T v = dx/dt = – x0 sin ωt = – v0 sin ωt a = dv/dt = – 2x0 cos ωt = – a0 cos t a = – 2x x0, v0, a0 positive maximum values v = ± x0 √1 - cos2 ωt = ± x0 √1 - x2 𝑥02 v = ± √x02 - x 2 EK = ½ m v2 = ½ m2 ( x02 - x 2 ) EK for x = 0 → EK(max) = ½ m2 x02 EK(max) = EP(max) = ETotal potential energy at any moment = total energy – KE EP = ½ m2 x2 Energy (total) of SHM is proportional to x02 Period does not depend on amplitude!!!!!! Damping: the presence of resistance forces on oscillations. That force is always in the opposite direction to the direction of motion of the oscillating particle and it is a dissipative force. "to damp" is to decrease the amplitude of an oscillation. Decreasing the amplitude doesn’t change period. Light/under damping: The decay in amplitude is relatively slow and the oscillator will make quite a few oscillations before finally coming to rest. Critical/heavy damping: occurs if the resistive force is so big that the system returns to its equilibrium position without passing through it. The mass comes to rest at its equilibrium position without oscillating. The friction forces acting are such that they prevent oscillations. Over-damping: the system returns to equilibrium without oscillations, but much slower than in the case of critical damping. Natural frequency is the frequency an object will vibrate with after an external disturbance. Forced oscillations: when an external periodic force with frequency fD is applied on a free system with a natural frequency f0 , the system may respond by switching to oscillations with a frequency equal to the driving frequency fD. Variation of the amplitude of vibrations of an object subjected to the forced frequency close to its natural frequency of vibration. Qualitative description of the factors that affect the frequency response and sharpness of the curve. ▪ For a small degree of damping, the peak of the curve occurs at the natural frequency of the system. ▪ The lower the degree of damping, the higher and narrower the curve. ▪ At very heavy damping, the amplitude is essentially constant. Resonance is increase in amplitude of oscillation of a system exposed to a periodic driving force with a frequency equal to the natural frequency of the system. ▪ Useful: microwave oven, radio. . ▪ Harmful: bridges, aero plane wings, internal organs in the case of heavy machinery . WAVES When a wave (energy) propagates through a medium, oscillations of the particles of the medium are simple harmonic. Progressive waves transfer energy through a distortion that travels away from the source of distortion. There is no net transfer of medium. ▪ Transverse waves are waves in which the particles of the medium oscillate perpendicular to the direction in which the wave is traveling. ◌ EM waves: light, radio waves, microwaves: need no medium, electric and magnetic field oscillate perpendicular to each other and to the direction of wave propagation. ◌ Earthquake secondary waves, waves on a stringed musical instrument, waves on the rope. ◌Transverse wave can not propagate in a gas ( and actually pure transverse can not propagate in liquid either) ▪ Longitudinal waves are waves in which the particles of the medium vibrate parallel to the direction in which the wave is traveling. ◌ Sound waves in any medium, shock waves in an earthquake, compression waves along a spring A wavefront is set of points having the same phase/displacement. A ray is an arrow drawn on a diagram to show the direction of propagation of waves. It is always at right angles to the wavefront. Energy of a wave of amplitude A is proportional to the amplitude2 : E ∞ A2 Although the speed of a wave depends only on the medium, there is a relationship between wavelngth 𝜆 , frequency f (period T) and the speed: v= = f T Waves that need medium to travel through are called mechanical waves. Electromagnetic wave is made up of changing electric and magnetic fields perpendicular to each other and to the propagation of the wave. They travel through vacuum with the SAME SPEED! speed of light c ≈ 3 x 108 m/s Index of refraction n,. of a medium is the ratio of the speed of light in a vacuum, c, and the speed of light, v, in that medium: c n= As the speed of light in air is almost equal to c, nair ≈ 1 v Refraction: When a wave passes from one medium to another, its speed changes resulting in a change in direction of the refracted wave. Snell’s law states that for a given pair of media, the ratio of the sines of the angles of incidence and refraction is equal to the ratio of velocities in the two media sinθ1 sinθ2 n = n2 = 1 v1 v2 = λ1 𝑓 λ2 𝑓 = λ1 λ2 v = 𝜆 f ; frequency doesn’t change in refraction, so in the medium with smaller wave speed, wavelength will be smaller. The refracted ray is refracted more in the medium with greater n, slower speed of light Chromatic dispersion is phenomenon in which the index of refraction depends on wavelength/frequency, so the speed of light through a material varies slightly with the frequency of the light and each λ is refracted at a slightly different angle. The longer λ, the smaller index of refraction. nred < nblue , red light is refracted less than blue light Dispersion is the phenomenon which gives separation of colours in prism/rainbow and undesirable chromatic aberration in lenses. Diffraction is the spreading of a wave into a region behind an obstruction (into a region of geometrical shadow). Diffraction effects are more obvious when wavelength of the wave is similar in size to aperture/obstacle or bigger. remember: big λ (compared to aperture or obstacle), big diffraction effects Interference is the addition (superposition) of two or more waves overlapping that results in a new wave pattern. Principle of superposition: When two or more waves overlap, the resultant displacement at any point and at any instant is the vector sum of the displacements of the individual waves at that point: y = y1 + y2 PD = path difference is the difference in distances traveled by waves from two sources to a point P: PD = d2 – d1 Two coherent waves traveling along two different paths to the same point will: interfere constructively if there is a difference in distance traveled that is equivalent to a whole number of wavelengths: PD = n λ n = 0, ± 1, ± 2, ± 3, … interfere destructively if there is a difference in distance traveled that is equivalent to a half number of wavelengths: PD = (n + ½ ) λ n = 0, ± 1, ± 2, ± 3, … Rutherford/Planetary/Nuclear Model of atom: the atom consists of a very tiny but very massive positive nucleus, surrounded by electrons that orbit the nucleus as result of electrostatic attraction between the electrons and the nucleus. evidence: Geiger and Marsden’s experiment: • Alpha particles bombarded at a sheet of gold foil mostly passed through—atoms mostly consist of empty space. • Particles that were deflected bounced straight back from the foil—the huge deflection of the alpha particles must have been caused by electrostatic repulsions between the positive alpha particles and a dense, positive nucleus. limitations/problems: • According to Maxwell, any accelerating charge will generate an EM wave • This EM wave would be a release of energy and give off light at all sorts of wavelengths. • electron releasing energy should slow down and eventually spiral into the nucleus . Bohr’s Model: • Electrons orbit at specific energy levels (“discrete states”), called “stationary states.” – we say energy of is quantized • Electrons in these stationary states do not emit EM waves as they orbit. • Photon must be first absorbed in order to be emitted. Absorbed photon has the energy equal to the difference between excited and ground state. • Photon is emitted when an electron jumps from excited state to a lower energy state. Energy of that photon is equal to the energy difference between two states. E photon = ΔE, and frequency is given by Einstein’s relationship between energy and frequency of a photon: E = hf h = Planck's constant = 6.627x10-34 Js Modern approach - Schrodinger wave function: • energy level model - give possible energies of electrons and probability to find electrons somewhere is given by wave function. Continuous Spectrum (without prism white light) • a spectrum having all wavelengths over a comparatively wide range • All possible frequencies of EM waves are present. • Generally, solids, liquids, or high pressured (dense) gases emit a continuous spectrum when heated. Discrete/ Line spectrum - Evidence of electron energy levels – Pattern of distinct lines of color, corresponding to particular wavelengths. • Emission Spectrum • Set of frequencies of the electromagnetic waves emitted by atoms of a particular element. • A hot, low-density / low pressure gas (gas in the atomic state) produces an emission-line spectrum – energy only at specific λ. • Absorption Spectrum • Pattern of dark lines against a continuous spectrum background that results from the absorption of selected frequencies by an atom or molecule. • An absorption spectrum occurs when light passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies; since the re-emitted light is unlikely to be emitted in the same direction as the absorbed photon, this gives rise to dark lines (absence of light) in the spectrum. Nucleon: a proton or neutron. Nuclide: A particular combination of protons and neutrons that form a nucleus. It is used to distinguish isotopes among nuclei. • X is chemical symbol of the element • Z is the atomic number = number of electrons or protons A ZX • A is the nucleon (mass) number = number of neutrons + protons • A – Z = number of neutrons Isotopes: Nuclides contains the same amount of protons but different number of neutrons. Isotopes are evidence for the existence of neutrons Repulsive electromagnetic forces between the protons would cause the nucleus to disintegrate if it were the only force. Strong nuclear force is an attractive force, which exists between all nucleons to hold them together. It is effective only over a very short range. Weak nuclear force exists only in the nucleus and is responsible for the disintegration of a neutron into a proton and an electron in beta decay. Nuclear Stability: depends on the neutron-proton ratio • Nuclei are held together by a strong nuclear force, which counteracts the repulsive force among protons contained within it. As long as the attractive nuclear forces between all nucleons win over the repulsive Coulomb forces between the protons the nucleus is stable. It happens as long as the number of protons is not too high. Atomic nuclei are stable subject to the condition that they contain an adequate number of neutrons, in order to "dilute" the concentration of positive charges ♦ Small nuclei- tend to have equal number of neutrons and protons ♦ Large nuclei- tend to have more neutrons to counterbalance repulsive Coulomb force. The most massive isotope found in nature is uranium isotope 238 . For more massive nuclei strong nuclear force can’t overcome electric repulsion. 92 U Radioactive Decay – Spontaneous decay of unstable nuclei. • process in which unstable nucleus loses energy by emitting “radiation” in form of particles or EM waves, resulting in transformation of parent nuclide into daughter nuclide. • three common radiations - alpha, beta, gamma • they differ in charge, ionization and penetration power. Alpha decay: nucleus ejects an α particle, the atomic number is decreased by two and the atomic mass is decreased by four • charge is + 2e • the most ionizing and therefore the least penetrating (a few cm of air)• Governed by strong nuclear force = α decay occurs primarily among heavy elements because the nucleus has too many protons which cause excessive repulsion. In an attempt to reduce the repulsion, a helium nucleus is emitted. Mass of parent > mass of daughter + mass of alpha • difference = kinetic energy Beta decay: nucleus spontaneously emits beta particle and an antineutrino • the most common decay occurs when the neutron to proton ratio is too great in the nucleus and causes instability • In β− decay, the weak interaction converts a neutron into a proton while emitting an electron and an anti-neutrino: 10n → 11p + −10e (β− ) + ν̅ : • charge is – e • medium ionizing and therefore medium penetrating (a few mm of metal) Gamma Decay – EM waves (high-energy photons) are emitted from a nucleus in an excited state dropping to a lower energy state (more stable) • charge is 0 • no ionizing and therefore highly penetrating (a few cm of lead) Biological effects of ionizing radiation: • Prompt effects: effects, including radiation sickness and radiation burns, seen immediately after large doses of radiation delivered over short periods of time. • Delayed effects: effects such as cataract formation and cancer induction that may appear months or years after a radiation exposure Radioactive Decay is a random process on the atomic level, in that it is impossible to predict when a particular atom will decay, but given a large number of similar atoms, the decay rate, on average, is predictable. The rate of decay decreases exponentially with time. Half – life T 1 /2 is the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay. Activity (becquerel - Bq) of a radioactive sample is the average number of disintegrations per second. Nuclear Reactions – A reaction that occurs whenever the number of protons or neutrons changes. Nuclear reactions include natural and artificial transmutation, fission, and fusion. Transmutation – Change of one element into another. In natural transmutations the nucleus decays spontaneously. There is only one nucleus that undergoes the transformation. Artificial transmutation is induced by the bombardment of the nucleus by high-energy particles (Uranium atoms bombarded with neutrons to start fission reaction.) Unified Atomic Mass Unit (u) is 1/12 of the mass of one atom of carbon-12 atom (6p+6n+6e) • 1 u = 1.66056655 x 10-27 kg 1 u of mass converts into 931.5 MeV (due to relationship E = mc2) Mass Defect (δ) is the difference between the total mass of all nucleons in the nucleus and the mass of the nucleus itself δ = Zmp + Nmn - Mnucleus (can be calculated in kg or u) . Equivalent to binding energy. Binding Energy is the work required to completely separate the nucleons of a nucleus/ energy released when nucleons form a nucleus. nuclear binding energy is actually energy that corresponds to mass defect 1. BE in MeV: find mass defect in u and multiply it by 931.5 MeV 2. ME in J: BE = δc2 (c = 3x108 m/s, δ = mass defect in kg) In order to balance nuclear reaction the total mass/energy and total charge number of the reactants has to equal the total mass/energy and total charge number of the products. Energy released/required in a nuclear reaction/artificial transmutation Nuclear reactions A + B → C + D can either release energy or requires energy input. • release energy: Energy will be released in nuclear reaction if Δm = LHS – RHS > 0 The total amount of energy released will be in the form of kinetic energy of products. If there was initial kinetic energy, that will be added up to released energy. energy released in nuclear reaction is found the same way as binding energy: first find mass difference and then equivalent energy Δm in u, E = (Δm) x 931.5 (MeV) or Δm in kg, E = (Δm) c2 (J) • energy input: if Δm = LHS – RHS < 0, reaction cannot be spontaneous. For example, some nuclei will decay only if energy is supplied to it - collision with fast moving α particle: α particle must have enough KE to make up for imbalance in masses, and to provide for kinetic energy of products. Energy released in a decay - conservation of total energy (energy + mass). as always 226 88 Ra 222 86 Rn 4 2 M > m1 + m2 , but total energy on the left = total energy on the right Mc2 = m1 c2 + m2 c2 + KE1 + KE2 • spontaneous decay: M > m1 + m2 → binding energy of the decaying nucleus < binding energies of the product nuclei. This is why radioactive decay happens with heavy elements lying to the right of maximum in the binding energy curve. Energy released is in the form of kinetic energy of the products. Binding energy per nucleon: the work required to remove one nucleon from the nucleus; roughly the binding energy divided by the total number of nucleons in nucleus. The binding energy of a nucleus is a measure of how stable nucleus is. Greater mass defect – higher binding energy – greater stability. Most nuclei have a binding energy per nucleon of approximately 8 MeV. Nuclear fission: process in which a large nucleus (A>200) splits up into two smaller nuclei, generally accompanied by the release of one or more neutrons and energy (as gamma rays and as kinetic energy of the fragments). Large amounts of energy produced, can be self-sustaining due to chain reactions. The total BE would increase which means that the daughters are more stable than parent. Spontaneous fission is very rare. Nuclear fusion: joining of two small nuclei into a bigger one, releasing great amounts of energy in the process. High temperatures are required to provide sufficient kinetic energy to approach each other, overcoming electrostatic repulsion. When two small nuclei the product of fusion would have more BE per nucleon. The increases in binding energy per nucleon are much larger for fusion than for fission reactions, because the graph increases more steeply for small nuclei, so fusion gives out more energy per nucleon than fission. Wind generator. air density ρ, wind speed v, area of the turbine A assumption: wind is stopped by the wind turbine, which is not the case, so not all of Ek of the wind is turned into electricity. Wave power: P/L amplitude of the wave A, length of wavefront L, - density of water Intensity I of the Sun’s radiation incident on a planet at distance r from the Sun is the power radiated received at distance r per unit area Astrophysics: apparent brightness b The power from the star received (incident) per m2 of the Earth’s surface. If the energy radiated by a star is emitted uniformly in all directions, then apparent brightness is b= L 4π𝑑2 where L is luminosity (power radiated) of the star and d its distance from the Earth. Albedo: Some of the radiation received by a planet is reflected straight back into space. The fraction that is reflected back is called the albedo, α= total (reflected) scaterred power total incident power Earth’s albedo varies daily and is dependent on season (cloud formations) and latitude. Oceans have a low value but snow has a high value. The global annual mean albedo is 0.3 (30%) on Earth. If the temperature of a planet is constant, then the power being absorbed by the planet must equal the rate at which energy is being radiated into space. The planet is in thermal equilibrium. Surface heat capacity is the energy required to raise the temperature of unit area of a planet’s surface by one degree, and is measured in J m-2 K-1 If the incoming radiation power and outgoing radiation power are not equal, then the change of the planet’s temperature in a given period of time is: ΔT = CS = energy area of surface x temperature change of surface (incoming radiation intensity - outgoing radiation intensity)× time Cs A black body is a theoretical object that absorbs all incident electromagnetic radiation. Therefore it reflects no radiation and appears perfectly black. It is also a perfect emitter of radiation. It would emit at every wavelength of light, and the “black body radiation” distribution as a function of wavelength, known as Planck’s law, depends upon its temperature. Although stars and planets are not perfect emitters, their radiation spectrum is approximately the same as black-body radiation. WIEN’S LAW STEFAN - BOLTZMANN LAW wavelength at which the intensity of the radiation is a maximum, λmax, is inversely to the temperature of the black body max (m) 2.9×10-3 T(K) The total power ((total energy per unit time) radiated by a black body is proportional to 4th power of surface temp. (astrophysics: luminosity) P = σAT4 = Stephan - Boltzmann constant A – surface area of the emitter T – absolute temperature of the emitter (in Kelvin) The Earth and its atmosphere are not a perfect black body. Emissivity, e, is defined as the ratio of power radiated by an object to the power radiated by a black body at the same temperature. e= power radiated by an object power radiated by black body at the same temperature Short wavelength radiation is received from the sun and causes the surface of the Earth to warm up. The Earth will emit infra-red radiation (longer wavelengths than the radiation coming from the sun because the Earth is cooler than the sun). Some of this infrared radiation is absorbed by gases in the atmosphere and re-radiated in all directions. Temperature of the Earth’s surface will be constant if the rate at which it radiates energy equals the rate at which it absorbs energy. The greenhouse effect is the warming of a planet due its atmosphere allowing in ultraviolet radiation from the Sun, but trapping the infrared radiation emitted by the warm Earth. Hydroelectric power gravitational PE of water → KE of water → KE of turbines → electrical energy The energy stored in a lake at altitude is gravitational PE = mgh. h is the height difference between the outlet from the lake and the turbine. Since not all of the water in the lake is the same height, the average height is used (this is assuming the lake is rectangular in cross section). The rate of change of the potential energy converted into kinetic energy is P= mgh t = (ρV)gh t = ρ Vt gh = ρ Q g h ρ – density of the water V – volume of the lake Q is known as the volume flow rate (m3/s ) There are only two ways to transfer energy from one body to another — either by doing work or by transferring thermal energy. Thermal energy may be completely converted to work in a single process, but that continuous conversion of this energy into work requires a cyclical process (use of machines that are continuously repeating their actions in a fixed cycle) and the transfer of some energy from the system (to the surroundings and therefore no longer available to perform useful work). Degraded energy is energy that has become less useful (unavailable), i.e. cannot perform mechanical work due to being transformed into other forms of energy, e.g. thermal energy (in accordance with the second law of thermodynamics) Sankey diagrams are used to represent different ways of producing useful energy. Fuel is a substance that can release energy by changing its chemical or nuclear structure. All possible sources of energy: ▪ The Sun’s radiated energy ▪ Gravitational energy of the Sun and the Moon ▪ Nuclear energy stored within atoms ▪ The Earth’s internal heat energy ○ The Sun is the prime energy source for the world’s energy. Energy density is the amount of energy that can be extracted per kilogram of fuel. Unit: J kg-1 Chain reaction: ▪ Energy is required to split a U – 236 nucleus. This can be supplied by adding a neutron to the U –235 nuclei, which destabilizes the nucleus U – 236 (formed after a neutron is caught by U – 235) and causes it to split in two. ▪ Extra neutrons are produced, which can go on to react with other U – 235 nuclei in a self-sustaining chain reaction. However neutrons must be first slowed down to less than 1 eV. Too fast neutrons are not likely to make reaction. Critical mass: the minimum mass required for a chain reaction. (atomic bomb: mass > critical mass) Fuel enrichment: ▪ Uranium comes naturally as 99.3% U-238. However only U – 235 is used in the reaction process. ▪ The process of increasing the percentage of U-235 in the material to make nuclear fission more likely is called enrichment. ▪ 3% U-235 must be reached in order to power a nuclear reactor. Controlled nuclear fission (power production) and uncontrolled nuclear fission (nuclear weapons) Main energy transformations in a nuclear power station: nuclear energy → thermal energy → mechanical energy → electrical energy Three important components in the design of all nuclear reactors are moderator, control rods and heat exchanger. ▪ Moderator is a medium that slows down fast neutrons to make them suitable for reaction (water, graphite, heavy water). ▪ Control rods are movable rods that readily absorb neutrons. They can be introduced or removed from reaction chamber in order to control the rate of fission of uranium and plutonium. Made of chemical elements capable of absorbing many neutrons without fissioning themselves (cadmium, hafnium, boron, etc) ▪ Heat exchanger is used to seal off the place where nuclear reactions take place from the rest of the environment. In some nuclear power plants, the steam from the reactor goes through a heat exchanger to convert another loop of water to steam, which drives the turbine. The advantage to this design is that the radioactive water/steam never contacts the turbine. Neutron capture by a nucleus of uranium-238 results in the production of a nucleus of plutonium-239 In addition to uranium – 235, plutonium – 239 is also capable of sustaining fission reactions. This nuclide is formed as a by - product of a conventional nuclear reactor. A uranium – 238 nucleus can capture fast moving neutrons to form uranium – 239. This undergoes β – decay to neptunium – 239 which undergoes further undergoes further β – decay to plutonium – 239: Plutonium-239 is used as a fuel in 238 U 92 + 10𝑛 → 239 92U 239 92 U → 239 Np 93 + −10𝛽 + 𝜈̅ 239 93 Np → 239 Pu 94 + −10𝛽 + 𝜈̅ other types of reactors. Problems associated with producing nuclear power using nuclear fusion: the reaction requires creating temperatures high enough to ionize atomic hydrogen into a plasma state. Currently the principal design challenges are associated with maintain and confining the plasma at sufficiently high temperature and density for fusion to take place. Solar Power ▪ Solar panel (active solar heater) is used for central heating or for making hot water for household use, placed on roofs of houses, consisting of metal absorber, water pipes, and glass. It converts solar energy into thermal energy of water. ▪ A photovoltaic cell converts solar radiation into electrical energy. Produces very small voltage. Option E: Astrophysics L = AT4 𝜆max(meters) = d(parsec) = 2.90×10-3 T (kelvin) 1 p (arc-second) L b = 4𝜋𝑑 2 m – M = 5 log ( d ) 10 Constellation is a group of stars that form a pattern as seen from the Earth, but not bound by gravitation. Stellar cluster is a group of stars held together by gravitation in same region of space, created roughly at the same time. Galaxy is a huge group of stars, dust, and gas held together by gravity, often containing billions of stars, measuring many light years across. Star is a massive body of gas held together by gravity, with fusion going on at its center, giving off electromagnetic radiation. There is an equilibrium between radiation pressure and gravitational pressure. Stars’ and planets’ radiation spectrum is approximately the same as black-body radiation/ Plank’s law. Intensity as a function of wavelength depends upon its temperature Wien’s law: Wavelength at which the intensity 2.9×10-3 of the radiation is a maximum λmax, is: max (m) T(K) Luminosity (of a star) is the total power (total energy per second) radiated by an object (star). If we regard stars as black body, then luminosity is: L = A σT4 = 4πR2σT4 (Watts) Stefan-Boltzmann’s law A is surface area of the star, R is the radius of the star, T surface temperature (K), σ is Stefan-Boltzmann constant. (Apparent) brightness (b) is the power from the star received per square meter of the Earth’s surface b= L 4π𝑑2 (W/m2) L is luminosity of the star; d its distance from the Earth Magnitude Scale • Magnitudes are a way of assigning a number to a star so we know how bright it is Apparent magnitude (m) of a celestial body is a measure of its brightness as seen from Earth. The brighter the object appears the lower its apparent magnitude. Greeks ordered the stars in the sky from brightest to faintest… Later, astronomers accepted and quantified this system. • Every one step in magnitude corresponds to a factor of 2.51 change in brightness. Ex: m1 = 6 and m2 = 9, then b1 = (2.51)3 b2 Absolute magnitude (M) of a star is the apparent magnitude that a star would have if it were at distance of 10 pc from Earth. It is the true measurement of a star’s brightness seen from a set distance. m – M = 5 log ( d ) 10 m – apparent magnitude M – absolute magnitude of the star d – its distance from the Earth measured in parsecs. • If two stars have the same absolute magnitude but different apparent magnitude they would have the same brightness if they were both at distance of 10 pc from Earth, so we conclude they have the same luminosity, but are at different distances from Earth !!!!!!!!!!!!!! • Every one step in absolute magnitude corresponds to a factor of 2.51 change in luminosity. Ex: M1 = – 2 and M2 = 5, then L1 / L2 = (2.51)7 Binary star is a stellar system consisting of two stars orbiting around their common center of mass. The ONLY way to find mass of the stars is when they are the part of binary stars. Knowing the period of the binary and the separation of the stars the total mass of the binary system can be calculated (not here). Visual binary: a system of stars that can be seen as two separate stars with a telescope and sometimes with the unaided eye. They are sufficiently close to Earth and the stars are well enough separated. Sirius A, brightest star in the night sky and its companion first white dwarf star to be discovered Sirius B. Spectroscopic binary: A binary-star system which from Earth appears as a single star, but whose light spectrum (spectral lines) shows periodic splitting and shifting of spectral lines due to Doppler effect as two stars orbit one another. Eclipsing binary: (Rare) binary-star system in which the two stars are too close to be seen separately but is aligned in such a way that from Earth we periodically observe changes in brightness as each star successively passes in front of the other, that is, eclipses the other. The Hertzsprung–Russell (H – R) diagram(family portrait) is a scatter graph of stars showing the relationship between the stars' absolute magnitudes / luminosities versus their spectral types(color) /classifications or surface temperature. It shows stars of different ages and in different stages, all at the same time. L = sun luminosity = 3.839 × 1026 W main sequence stars: fusing hydrogen into helium, the difference between them is in mass: left upper corner more massive than right lower corner. white dwarf compared to a main sequence star: • has smaller radius • more dense • higher surface temperature • energy not produced by nuclear fusion Techniques for determining stellar distances: stellar parallax, spectroscopic parallax and Cepheid variables. Stellar parallax • two apparent positions of a close star with respect to position of distant stars as seen by an observer from two widely separated points are compared and recorded; • the maximum angular variation from the mean, p, is recorded; • the distance (in parsecs) can be calculated using geometry astronomical unit 1 AU Sun-earth distance tan p = = for small angles: tan θ ≈ sin θ ≈ θ (in radians) 1 AU = 1.5 x 1011 m d Sun-star distance d= 1 AU p if p = 1 sec of arc, d = 3.08x1016 m defined as 1 pc d (parsecs) = 1 p(arcseconds) limit because of small parallaxes: d ≤ 100 pc Spectroscopic parallax: no parallax at all!!!! (a lot of uncertainty in calculations) • light from star analyzed (relative amplitudes of the absorption spectrum lines) to give indication of stellar class/temperature • HR diagram used to estimate the luminosity • distance away calculated from apparent brightness limit: d ≤ 10 Mpc Cepheid variables are stars with regular variation in absolute magnitude (luminosity) (rapid brightening, gradual dimming) which is caused by periodic expansion and contraction of outer surface (brighter as it expands). This is to do with the balance between the nuclear and gravitational forces within the star. In most stars these forces are balanced over long periods but in Cepheid variables they seem to take turns, a bit like a mass bouncing up and down on a spring. There is a clear relationship between the Left: graph shows how the apparent period of a Cepheid variable and its absolute magnitude. The greater the period magnitude (the brightness) changes, . then the greater the maximum luminosity getting brighter and dimmer again of the star. Cepheids typically vary in with a fixed, measurable period for a brightness over a period of about 7 days. particular Cepheid variable. Left is general luminosity – period graph. So, to find out how far away Cepheid is: • Measure brightness to get period • Use graph absolute magnitude M vs. period to find absolute magnitude M • Measure maximum brightness • Calculate d from b = L/4πd2 • Distances to galaxies are then known if the Cepheid can be ascertained to be within a specific galaxy. Newton assumptions about the nature of the universe: • universe is infinite in extent • contains an infinite number of stars uniformly distributed • is static and exists forever • these assumptions led to Olber’s paradox Olber’s paradox density of stars n = N/V = number of stars per unit volume • divide the whole universe into concentric shells around Earth of constant thickness t • look at one shell of thickness t at distance d from the Earth • since stars are uniformly distributed the number of stars seen from Earth increases as d2: number of stars in shell = density x volume = n 4πd2 t • brightness of one star decreases as 1/d2 ; b = L/4 πd2 • brightness of shell is constant; assuming that luminosity L is the same for all stars, the received energy per sec per unit area (brightness) from all stars in the thin shell is: L 4π d2 × 4π d2 nt = Lnt = const. • amount of light we receive from shell does not depend upon how far away the shell is • adding all shells to infinity; each contributing a constant amount of energy • the total energy is infinite • sky would be uniformly bright • but it’s dark in night Solutions to Olber’s paradox • Perhaps the Universe is not infinite. But current model of the Universe is that it is infinite. • Perhaps the light is absorbed before it gets to us. But then Universe would warm up and eventually reradiate energy. Real help: the Big Bang model leads to the idea that the observable universe is not infinite and to the idea of the expansion of the universe; • Universe is not static, it is expanding, hence the most distant stars/ galaxies are strongly red - shifted, out of the visible part of the spectrum. • There is a finite time since the Big Bang. Some 12 to 15 billion years. That means we can only see the part of it that lies within 12 to 15 billion light-years from us. And the observable part of the universe contains too few stars to fill up the sky with light. Calculation shows that the helium produced by nuclear fusion within stars cannot account for the real amount of helium in Universe (24%). In 1960 it was proposed that sometime during the early history of the Universe, long before any star, Universe was at a sufficiently high temperature to produce helium by fusion. In this process many high energy photons would be produced. The CMB (Cosmic Microwave Background Radiation) radiation was emitted only a few hundred thousand years after the Big Bang, long before stars or galaxies ever existed. The photons would have a black body spectrum corresponding to the then temperature of the Universe. As the Universe expanded and cooled the photon spectrum would also change with their maximum wavelength shifting in accordance with Wien’s law. It is estimated that at the present time the photons should have a maximum wavelength corresponding to a black body spectrum of an extremely cold object of temperature of 2.7 K. Cosmological background radiation / Cosmic microwave background radiation (CMB) is microwave radiation - left over from the Big Bang that fills the universe roughly uniformly in all directions. The Big Bang predicts an expanding universe that had a very high temperature at the beginning; during the expansion the universe is cooling down and the temperature of the radiation should fall to its present low value of about 2.7 K. That radiation corresponds to a black body spectrum of about 2.7 K. The other way of explaining CMB is: Big Bang producing initially produced very short wavelength photons /EM radiation. As the universe expands, the wavelengths become red shifted to reach current value. █ Explain how knowledge of the spectrum of a black body and the existence of cosmological background radiation is consistent with the “Big Bang” model of the universe. ► Big Bang predicts a low temperature radiation at 2.7 K (i.e. CMB radiation). The Big Bang theory also predicts an expanded universe which we observe through red- shifting of the galaxies and the lowering of CMB radiation temperature. This expanding universe is the result of the initial energy released in the Big Bang █ State one piece of evidence that indicates that the Universe is expanding. ► light from distant galaxies/stars is red-shifted (which means they move away from us – as the red-shifting occurs in all direction, the universe must be expanding) ► existence of CMB ► the helium abundance in the universe which is about 25 % and is consistent with a hot beginning of the universe; The eventual fate of the Universe is determined by the amount of mass in the Universe. Critical density is the density of the Universe which produces a flat universe, i.e. it would take an infinite amount of time to stop expansion of it. Critical density is the density of the Universe that would be necessary to stop the expansion after an infinite amount of time. • Closed Universe A model of the universe in which density of the Universe is such that gravity will stop the universe expanding and then cause it to contract. Eventually the contraction will result in a ‘Big Crunch’ after which the whole creation process could start again. • Open Universe A model of the universe in which density is such that gravity is too weak to stop the Universe expanding forever. • Flat Universe means that the density is at a critical value whereby the Universe will only start to contract after an infinite amount of time. non-coincident starts (not at beginning); correct shapes and correctly labelled; coincident at appropriate place; Dark matter is the matter that makes up for most of the mass in the universe, but cannot easily be detected because it does not emit radiation . Examples of dark matter. ► two of Neutrinos / WIMPS / MACHOS / black holes / exotic super symmetric particles / grand unified predicted particles / magnetic monopoles etc.; or maybe our current theory of gravity is again not correct OPTION G - EM WAVES Chromatic dispersion is phenomenon in which the index of refraction depends on wavelength/frequency, so the speed of light through a material varies slightly with the frequency of the light and each λ is refracted at a slightly different angle. The longer λ, the smaller index of refraction nred < nblue , red light is refracted less than blue light Electromagnetic waves are produced by the accelerated electric charges. 1. radio waves: antenna: an alternating current produces a radio wave 2. light : When an electron changes from a high energy level to a low one it emits electromagnetic radiation/photon. Energy of the photon equals the electron’s change in energy ΔE. The frequency of the emitted light, f is given by: ΔE = hf, where h is Planck’s constant 6.63 x 10-34 Js 3. Even higher frequencies Electron energy levels are in the order of 10 eV. Much higher frequencies would need an energy change in the order of MeV, much greater than electron energies. Radiation with such high energy comes from the nucleus. • EM wave is a transverse wave, the electric and magnetic fields oscillate perpendicular to each other and also perpendicular to the direction of wave propagation, traveling through vacuum with the SAME SPEED! c ≈ 3 x 108 m/s, which is independent of the motion of the source. c = λf • EM waves carry energy (as any wave does) which is directly proportional to its frequency, inversely proportional to its wavelength. In addition, energy/ intensity of a wave is proportional to its amplitude2. When EM waves is incident upon some medium it will be transmitted, absorbed or scattered to different extend depending on both wavelength and medium involved. If the Earth had no atmosphere, we would see the Sun as white star in sea of blackness. Scattering is the process of absorption and reemission of a light wave in different direction. A day-time sky is blue because molecules in the air (the nitrogen and oxygen molecules...) scatter blue light from the sun more than they scatter red light. Blue light is scattered all around the sky. Whichever direction you look, some of this scattered blue light reaches you. At sunset sky is red. The path of the light through the atmosphere is longer, so there is more chance for the blue light to scatter multiple times, less chance to reach eye. When we look towards the sun at sunset, we see red and orange colours because the blue light has been scattered out and away from the line of sight. A monochromatic source of radiation is one that has a extremely narrow band of frequencies. Two sources are coherent if they ▪ have the same frequency ▪ maintain a constant phase difference with each other. Two coherent waves have a phase difference which remains constant over time. When a light bulb emits photons, they are emitted randomly in different directions and with different phase because the filament atoms act independently from each other. The light emitted is incoherent. Laser light is a coherent and monochromatic source of electromagnetic radiation. MASER Microwave amplification by stimulated emission of radiation LASER Light amplification by stimulated emission of radiation Both apply the same principles (stimulated emission, existence of metastable state and population inversion) and only differ in the frequency range. Spontaneous Emission: An atom in excited state returns to the lower state spontaneously. LED, lamp… Stimulated Emission: Suppose an electron is already in an excited state and a photon comes along with energy equal to the difference in the energy between the excited state and the ground state of the atom or molecule. Photon will stimulate the electron to fall into the lower energy state emitting a photon which is in phase and in the same direction as original photon. This is resonance process, called stimulated emission. One photon interacting with an excited atom results in two photons coherent photons (identical and in phase). Population inversion: When a sizable population of electrons resides in higher energy level, this condition is called a "population inversion". It is necessary to create a population inversion for laser action to occur. Some atoms will undergo spontaneous emission, and the resulting photons cause other atoms to undergo stimulated emission, leading to a chain reaction. The resultant light is composed of one frequency, very intense, and coherent. To have inverse population it is important that higher energy level is metastable state – excited state with longer lifetime, so the electrons can remain in this state for a longer period before they decay to the ground state. Young ’s double slit experiment constructive interference – bright fringe d sin = (n + ½ ) λ destructive interference – dark fringe ● intensity distribution of the fringes on the screen when the separation of the slits is large compared to their width. The fringes are of equal intensity and of equal separation. If the slit separation is decreased, the pattern will spread out. Multiple slits – diffraction grating • bright fringes maintain the same separation. • bright fringes become much sharper. • the overall amount of light being let through is increased, so the pattern increases in intensity. If white light is viewed through a diffraction grating, each wavelength undergoes constructive interference at different angles. This results in a spectrum. The individual wavelengths can be calculated from the angle using the formula d sin θ = n λ greater λ greater angle Lenses: • Optical axis – axis of symmetry of a lens • Focal point – the point to which light rays parallel to the optical axis converge after passing through a converging lens. It is a real image of an object at infinite distance from lens. Because light can go both ways lens has two focal points. Standard rays • ray parallel to optical axis is refracted through the lens through focal point F • ray coming through the centre of the lens will continue n the same direction • ray passing through F in front of the lens is refracted parallel to the axis image can be real/virtual, enlarged/diminished and in the same direction/inverted. Real image is formed when the light passes through the actual image location. Such image can be caught on the screen. Virtual image is image which is formed at the position where extended rays cross. For the eyes (and brain) it seems as if these refracted rays were coming from virtual image – intersection of the extended rays. The Thin-Lens Equation focal length f is + for a converging lens u is distance between lens and object: v is distance between lens and image: Linear magnification m= image height object height real object, image: positive u, v virtual object, image: negative u, v Power of a lens (P) – measure of the extent of refraction of light: P (diopters) = 1 f (m) object, image upward: positive ho, hi object, image downward: negative ho, hi f greater – less curvature – less refraction – smaller P f smaller – more curvature – more refraction – greater P Optics of the eye: Near Point = distance of the closest object that can be focused on the retina: D ≈ 25 cm Far point = distance of the farthest object that can be focused on the retina = ~ infinity Angular magnification of a simple microscope (magnifying glass) M= angle subtended at eye using instrument angle subtended at unaided eye telescope magnification unaided eye: the greatest possible apparent size will be if the object is at near point . If the object is closer, the image is not clear, if it is farther apparent size is smaller. for virtual image formed by lens at eye’s near point, angular magnification is: M= D +1 f If the object is placed at focal point of the lens, virtual image is formed at infinity. Eye is relaxed. angular magnification is: M= D f COMPOUND MICROSCOPE – normal adjustment A compound microscope consists of two lenses — the objective lens and the eyepiece lens. The first lens (the objective lens) forms a real magnified image of the object being viewed. This real image can then be considered as the object for the second lens (the eyepiece lens) which acts as a magnifying glass. The rays from this real image travel into the eyepiece lens and they form a virtual magnified image. In normal adjustment, this virtual image is arranged to be located at the near point so that maximum angular magnification is obtained. M = m1 m 2 angular magnification = linear magnification produced by eye piece x linear magnification produced by objective ASTRONOMICAL TELESCOPE – normal adjustment An astronomical telescope also consists of two lenses. In this case, the objective lens forms a real but diminished image of the distant object being viewed (no other choice). Once again, this real image can then be considered as the object for the eyepiece lens acting as a magnifying lens. The rays from this real image travel into the eyepiece lens and they form a virtual magnified image. In normal adjustment, this virtual image is arranged to be located at infinity. Angular magnification M = 𝑓o fe The length of the telescope ≈ fo + fe Difference between microscope and telescope: microscope: object is between F & 2 F of the objective first image is real, enlarged within focal length of the eyepiece, so the image formed by eyepiece is virtual and enlarged. The situation with telescope is similar except that the object is at infinite distance and image formed by the first lens is real and smaller. Aberrations In an ideal lens, all light rays from one point of the object would meet at the same point of the image, forming a clear image. The influences which cause different rays to converge to different points are called aberrations. Lenses do not form perfect images, and there is always some degree of distortion or aberration introduced by the lens which causes the image to be an imperfect replica of the object. Careful design of the lens system for a particular application ensures that the aberration is minimized. There are several different types of aberration which can affect image quality. (Wikipedia) Spherical Aberration occurs because spherical surfaces are not the ideal shape with which to make a lens, but they are by far the simplest shape to which glass can be ground and polished (the least expensive) and so are often used. perfect lens spherical lens paralel light rays striking the outer edges of a lens are focused in a slightly different place than beams close to the axis. This problem is not limited to parallel light. Any incident ray which strikes the outer edges of the lens is subject to this departure from the expected or proper course for the ideal lens. This manifests itself as a blurring of the image. Lenses in which closer-to-ideal, non-spherical surfaces are used are called aspheric lenses. Correction for spherical aberration this or money remember that all rays incident on the lens from the object will be focused, and that the image will be formed even if part of the lens is covered. The image will be simply dimmer. Chromatic Aberration A lens will not focus different colors in exactly the same place because the focal length depends on refraction and the index of refraction for blue light (short wavelengths) is larger than that of red light (long wavelengths). The amount of chromatic aberration depends on the dispersion of the glass. One way to minimize this aberration is to use glasses of different dispersion in a doublet or other combination This effect can be reduced by having a combination of a convex and a concave lens made of glasses having different refractive indices. Chromatic aberration can be minimized using additional lenses In an Achromat, the second lens cancels the dispersion of the first. Achromats use two different materials, and one has a negative focal length.