Radioactivity_Notes_Sections_21.1_
advertisement
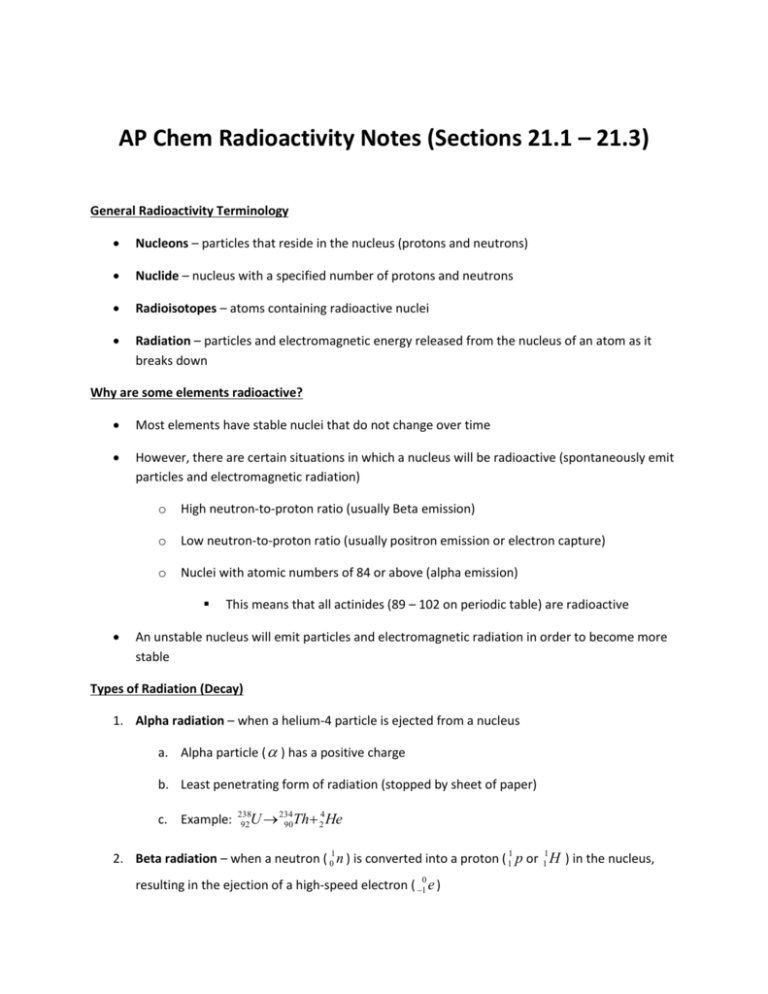
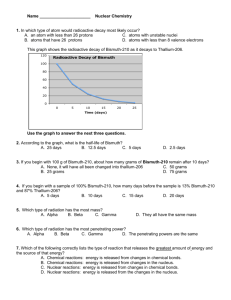
AP Chem Radioactivity Notes (Sections 21.1 – 21.3) General Radioactivity Terminology Nucleons – particles that reside in the nucleus (protons and neutrons) Nuclide – nucleus with a specified number of protons and neutrons Radioisotopes – atoms containing radioactive nuclei Radiation – particles and electromagnetic energy released from the nucleus of an atom as it breaks down Why are some elements radioactive? Most elements have stable nuclei that do not change over time However, there are certain situations in which a nucleus will be radioactive (spontaneously emit particles and electromagnetic radiation) o High neutron-to-proton ratio (usually Beta emission) o Low neutron-to-proton ratio (usually positron emission or electron capture) o Nuclei with atomic numbers of 84 or above (alpha emission) This means that all actinides (89 – 102 on periodic table) are radioactive An unstable nucleus will emit particles and electromagnetic radiation in order to become more stable Types of Radiation (Decay) 1. Alpha radiation – when a helium-4 particle is ejected from a nucleus a. Alpha particle ( ) has a positive charge b. Least penetrating form of radiation (stopped by sheet of paper) c. Example: 4 U 234 90Th 2 He 238 92 2. Beta radiation – when a neutron ( 01 n ) is converted into a proton ( 11 p or 11 H ) in the nucleus, resulting in the ejection of a high-speed electron ( 10 e ) a. Beta particle ( ) has a negative charge b. More penetrating form of radiation than alpha (stopped by thin piece of wood) c. Example: 131 53 0 I 131 54 Xe 1 e 3. Positron emission – a positron ( 10 e ) is ejected from the nucleus as the result of the conversion of a proton to a neutron a. a positron has the same mass as an electron but the opposite charge b. positrons have very short lives because they are annihilated when they collide with an electron c. Example: 116C 115B 10 e 4. Electron capture – electron from outside the nucleus is captured by the nucleus and is consumed, causing the conversion of a proton to a neutron a. Example: 81 37 Rb 10 e (orbital electron) 3681Kr 5. Gamma radiation – high energy photons (packets of electromagnetic radiation with very short wavelength); they CONTAIN NO CHARGE AND THEY HAVE NO MASS a. Gamma radiation does not change the mass number or the atomic number of the nucleus b. Gamma radiation almost always is the energy that results from one of the other types of radiation c. Gamma radiation ( ) does not have to be shown when writing nuclear equations d. Most penetrating type of radiation (can usually be stopped by lead or concrete) e. Therefore, lead is commonly used as a shield from radiation Nuclear Transmutations In addition to natural radioactive decay, nuclear reactions can be caused by a nucleus or a neutron striking another nucleus. This is done artificially (not a natural process). There are a couple ways to do this: o Using charged particles – this is how particle accelerators work. Charged particles are crashed into a nucleus at very high speeds designed to overcome the force of repulsion. o Using neutrons in nuclear reactors – this is a very common way to create radioactive isotopes used in research and medicine Writing Nuclear Equations The main thing to remember when writing a nuclear equation is that the sum of the mass numbers on the reactants side must equal the sum of the mass numbers on the product side (same is true for atomic number) Examples: o What product is formed when radium-226 undergoes alpha decay? o Write a nuclear equation for the decay of thorium-231 to form protactinium-231. o Write the nuclear equation for mercury-201 undergoing electron capture. o #24a on homework: o Write the nuclear equation for neutron bombardment of 58 26 Fe