Supplementary Table 1 | KATs Sub-family Enzyme Location Enzyme
advertisement
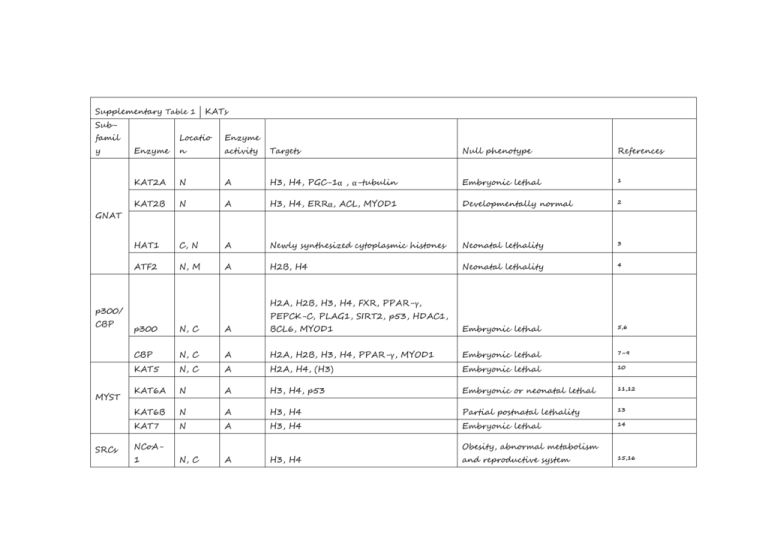
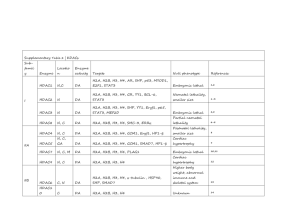
Supplementary Table 1 | KATs Subfamil y Locatio Enzyme Enzyme n activity Targets Null phenotype References KAT2A N A H3, H4, PGC-1α , α-tubulin Embryonic lethal 1 KAT2B N A H3, H4, ERRα, ACL, MYOD1 Developmentally normal 2 HAT1 C, N A Newly synthesized cytoplasmic histones Neonatal lethality 3 ATF2 N, M A H2B, H4 Neonatal lethality 4 GNAT H2A, H2B, H3, H4, FXR, PPAR-γ, p300/ CBP MYST SRCs PEPCK-C, PLAG1, SIRT2, p53, HDAC1, p300 N, C A BCL6, MYOD1 Embryonic lethal 5,6 CBP N, C A H2A, H2B, H3, H4, PPAR-γ, MYOD1 Embryonic lethal 7–9 KAT5 N, C A H2A, H4, (H3) Embryonic lethal 10 KAT6A N A H3, H4, p53 Embryonic or neonatal lethal 11,12 KAT6B N A H3, H4 Partial postnatal lethality 13 KAT7 N A H3, H4 Embryonic lethal 14 N, C A H3, H4 NCoA1 Obesity, abnormal metabolism and reproductive system 15,16 NCoA2 Smaller size, abnormal N, C A H3, H4 C, N A H3, H4 metabolism 18,19 NCoA3 17 Reduced bodyweight, abnormal TAFII25 Others reproductive system A, P, 0 N U-A H3, H4, (H2A), p53 Unknown 20 GTF3Cα N A H2A, H3, H4 Embryonic lethal 21 CLOCK N, C A H3, H4, GR, ARNTL1 Abnormal circadian phase 22,23 All references reported in this table correspond to the first known descriptions and mouse phenotyping studies for each genetic knockout model. Abbreviations: A, acetylation; ACL, ATP-citrate synthase; ARNTL1, aryl hydrocarbon receptor nuclear translocatorlike protein 1, also known as BMAL1 and MOP3; ATF2, cyclic AMP-dependent transcription factor ATF-2; C, cytosol; CBP, CREBbinding protein; CLOCK, circadian locomoter output cycles protein kaput; ERR-α, steroid hormone receptor ERR1; FXR, bile acid receptor, also known as farnesoid X-activated receptor; GR, glucocorticoid receptor, also known as NR3C1; GTF3C-α, general transcription factor 3C polypeptide 1, also known as TFIIIC220; HAT1, histone acetyltransferase type B catalytic subunit; KAT2A, histone acetyltransferase KAT2A (EC 2.3.1.48), also known as GCN5; KAT2B, histone acetyltransferase KAT2B, also known as PCAF; KAT5, histone acetyltransferase KAT5, also known as Tip60; KAT6A, histone acetyltransferase KAT6A, also known as MOZ and MYST3; KAT6B, histone acetyltransferase KAT6B, also known as MORF, MYST4 and MOZ2; KAT7, histone acetyltransferase KAT7, also known as HBO1 and MYST2; M, mitochondria; MYOD1, myoblast determination protein 1; N, nuclear; NCoA-1, nuclear receptor coactivator 1, also known as SRC-1; NCoA-2, nuclear receptor coactivator 2, also known as SRC-2; NCoA-3, nuclear receptor coactivator 3, also known as RAC-3, TRAM1 and AIB1; P, phosphorylation; p53, cellular tumour antigen p53; p300, histone acetyltransferase p300; PEPCK-C, phosphoenolpyruvate carboxykinase, cytosolic [GTP]; PLAG1, zinc finger protein PLAG1; PPAR-γ, peroxisome proliferator-activated receptor γ; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; SIRT2, NAD-dependent protein deacetylase sirtuin-2; TAFII250, transcription initiation factor TFIID subunit 1, also known as TAF1 and p250; α-tubulin, tubulin α-1A chain; U-A, ubiquitin-activating/conjugating. References: 1. 2. Xu, W. et al. Loss of Gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nat. Genet. 26, 229–232 (2000). Yamauchi, T. et al. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc. Natl Acad. Sci. USA 97, 11303–11306 (2000). 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. Nagarajan, P. et al. Histone acetyl transferase 1 is essential for mammalian development, genome stability, and the processing of newly synthesized histones H3 and H4. PLoS Genet. 9, e1003518 (2013). Maekawa, T. et al. Mouse ATF-2 null mutants display features of a severe type of meconium aspiration syndrome. J. Biol. Chem. 274, 17813–17819 (1999). Zheng, G. & Yang, Y.-C. Sumoylation and acetylation play opposite roles in the transactivation of PLAG1 and PLAGL2. J. Biol. Chem. 280, 40773–40781 (2005). Yao, T.P. et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93, 361–372 (1998). Tanaka, Y. et al. Abnormal skeletal patterning in embryos lacking a single Cbp allele: a partial similarity with Rubinstein–Taybi syndrome. Proc. Natl Acad. Sci. USA 94, 10215–10220 (1997). Kung, A.L. et al. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 14, 272–277 (2000). Zhang, Z., Hofmann, C., Casanova, E., Schütz, G. & Lutz, B. Generation of a conditional allele of the CBP gene in mouse. Genesis 40, 82–89 (2004). Gorrini, C. et al. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature 448, 1063–1067 (2007). Voss, A.K. et al. MOZ regulates the Tbx1 locus, and Moz mutation partially phenocopies DiGeorge syndrome. Dev. Cell 23, 652–663 (2012). Katsumoto, T. et al. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 20, 1321–1330 (2006). Thomas, T., Voss, A.K., Chowdhury, K. & Gruss, P. Querkopf, a MYST family histone acetyltransferase, is required for normal cerebral cortex development. Development 127, 2537–2548 (2000). Kueh, A.J., Dixon, M.P., Voss, A.K. & Thomas, T. HBO1 is required for H3K14 acetylation and normal transcriptional activity during embryonic development. Mol. Cell. Biol. 31, 845–860 (2011). Yamada, T. et al. SRC-1 is necessary for skeletal responses to sex hormones in both males and females. J. Bone Miner. Res. 19, 1452–1461 (2004). Xu, J. et al. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279, 1922–1925 (1998). Gehin, M. et al. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol. Cell. Biol. 22, 5923–5937 (2002). Liu, Z., Liao, L., Zhou, S. & Xu, J. Generation and validation of a mouse line with a floxed SRC-3/AIB1 allele for conditional knockout. Int. J. Biol. Sci. 4, 202–207 (2008). Wang, Z. et al. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl Acad. Sci. USA 97, 13549–13554 (2000). Li, H.-H., Li, A.G., Sheppard, H.M. & Liu, X. Phosphorylation on Thr-55 by TAF1 mediates degradation of p53: a role for TAF1 in cell G1 progression. Mol. Cell 13, 867–878 (2004). Hsieh, Y.J., Kundu, T.K., Wang, Z., Kovelman, R. & Roeder, R.G. The TFIIIC90 subunit of TFIIIC interacts with multiple components of the RNA polymerase III machinery and contains a histone-specific acetyltransferase activity. Mol. Cell. Biol. 19, 7697–7704 (1999). Doi, M., Hirayama, J. & Sassone-Corsi, P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 125, 497–508 (2006). Debruyne, J.P. et al. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 50, 465–477 (2006).



![Shark Electrosense: physiology and circuit model []](http://s2.studylib.net/store/data/005306781_1-34d5e86294a52e9275a69716495e2e51-300x300.png)