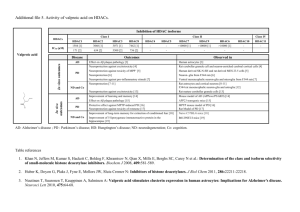
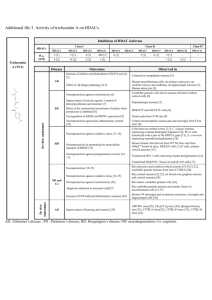
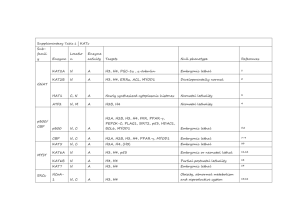
Supplementary Table 2 | KDACs Sub
advertisement
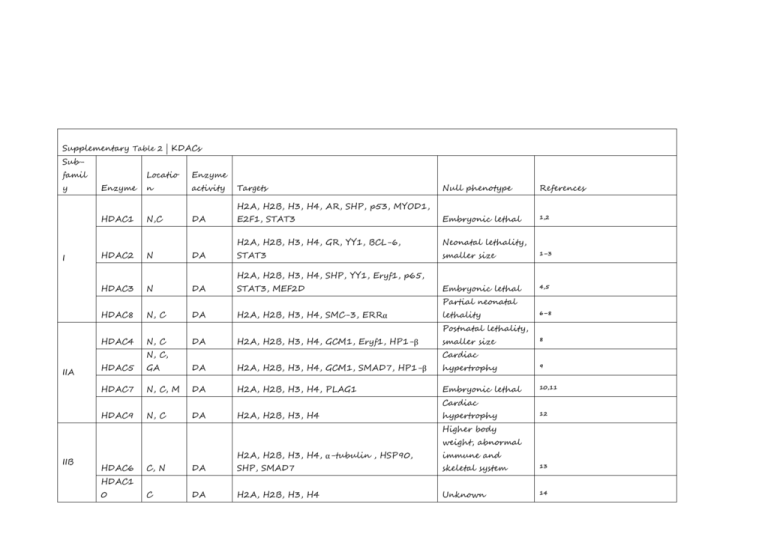
Supplementary Table 2 | KDACs Subfamil y Locatio Enzyme Enzyme n activity HDAC1 N,C DA HDAC2 N DA HDAC3 N DA Targets Null phenotype References E2F1, STAT3 Embryonic lethal 1,2 H2A, H2B, H3, H4, GR, YY1, BCL-6, Neonatal lethality, STAT3 smaller size 1–3 Embryonic lethal 4,5 H2A, H2B, H3, H4, AR, SHP, p53, MYOD1, I H2A, H2B, H3, H4, SHP, YY1, Eryf1, p65, STAT3, MEF2D Partial neonatal HDAC8 N, C DA H2A, H2B, H3, H4, SMC-3, ERRα lethality 6–8 Postnatal lethality, HDAC4 N, C DA H2A, H2B, H3, H4, GCM1, Eryf1, HP1-β N, C, IIA smaller size 8 Cardiac HDAC5 GA DA H2A, H2B, H3, H4, GCM1, SMAD7, HP1-β hypertrophy 9 HDAC7 N, C, M DA H2A, H2B, H3, H4, PLAG1 Embryonic lethal 10,11 HDAC9 N, C DA H2A, H2B, H3, H4 Cardiac hypertrophy 12 Higher body weight, abnormal IIB HDAC6 H2A, H2B, H3, H4, α-tubulin , HSP90, immune and C, N DA SHP, SMAD7 skeletal system 13 C DA H2A, H2B, H3, H4 Unknown 14 HDAC1 0 H2A, H3, H4, PGC-1-α , PPAR-γ, FOXO1, SIRT1 SIRT2 SIRT3 III N, C C M DA, DC DA, DC DA, DC FOXO3, CRTC2, AceCS, p53, NOTCH1, p65, Developmental HIF-1α, LXRβ, FXR defects H3, H4, ACL, α-tubulin, PEPCK-C, FOXO1, Developmentally p65 normal LCAD, HMGCS2, GDH1, SOD, IDH-2, Developmentally OXPHOS, AceCS2 normal 18,23,24 Developmentally 25–27 ADPR, SIRT4 M L, B GDH1, MCD, PDC 15–18 18–22 normal DA, DM, SIRT5 M, C, N DS, DG Developmentally CPS1 normal 28–30 H3, IGF-1, HIF-1α, KAT2A, p65 Premature aging 31–33 DA, ADPR, SIRT6 N D Smaller size, short lifespan, heart IV SIRT7 N HDAC1 N, C, 1 PM DA H3, GABPB-1, p53, SMAD6 defects 34,35 Developmentally DA H2A, H2B, H3, H4 normal 36 All references reported in this table correspond to the first known descriptions and mouse phenotyping studies for each genetic knockout model. Abbreviations: AceCS1, acetyl-coenzyme A synthetase, cytoplasmic, also known as ACSS2; AceCS2, acetyl-coenzyme A synthetase 2-like, mitochondrial; ADPR, ADP-ribosylation; B, biotinylation; BCL-6, B-cell lymphoma 6 protein; C, cytosol; CPS1, carbamoyl-phosphate synthase [ammonia], mitochondrial; CRTC2, CREB-regulated transcription coactivator 2; D, deacylation; DA, deacetylation; DC, decrotonylation; DM, demalonylation; DS, desuccinylation; DG, deglutaryation; E2F1, transcription factor E2F1; ERRα, steroid hormone receptor ERR1, also known as ESRRA; Eryf1, erythroid transcription factor, also known as GATA1; FOXO1, forkhead box protein O1; FOXO3, forkhead box protein O3; FXR, bile acid receptor, also known as farnesoid X-activated receptor; GA, golgi apparatus; GABPB-1, GA-binding protein subunit β1; GCM1, chorion-specific transcription factor GCMa; GDH1, glutamate dehydrogenase 1, mitochondrial (GDH 1); GR, glucocorticoid receptor, also known as NR3C1; HDAC1-11, histone deacetylase 1-11; HIF-1α, hypoxia-inducible factor 1α; HMGCS2, hydroxymethylglutaryl-CoA synthase, mitochondrial (HMG-CoA synthase); HP1-β, chromobox protein homolog 1; IDH-2, isocitrate dehydrogenase [NAD] regulatory subunit 2, mitochondrial; IGF-1 insulin-like growth factor I; L, lipoylation; LCAD, long-chain specific acyl-CoA dehydrogenase, mitochondrial; LXRβ, oxysterols receptor LXR-β; M, mitochondria; MCD, malonyl-CoA decarboxylase, mitochondrial (MCD); MEF2D, myocyte-specific enhancer factor 2D; MYOD1, myoblast determination protein 1; N, nuclear; NOTCH1, neurogenic locus notch homolog protein 1; p53, cellular tumour antigen p53; p65, transcription factor p65; PDC, pyruvate dehydrogenase complex; PEPCK-C, phosphoenolpyruvate carboxykinase, cytosolic [GTP]; PLAG1, zinc finger protein PLAG1; PM, plasma membrane;. SHP, Nuclear receptor subfamily 0 group B member 2; SIRT1-7, NADdependent protein deacetylase sirtuin-1-7; SMAD7, mothers against decapentaplegic homolog 7; SMC-3, structural maintenance of chromosomes protein 3; SOD, superoxide dismutase; STAT3, signal transducer and activator of transcription 3; α-tubulin, tubulin α1A chain; YY1, transcriptional repressor protein YY1, also known as EF-E1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Ref eren ces: 1. Ya mag uchi , T. et al . Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 24, 455–469 (2010). Montgomery, R.L. et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 21, 1790–1802 (2007). Guan, J.-S. et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459, 55–60 (2009). Bhaskara, S. et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol. Cell 30, 61–72 (2008). Montgomery, R.L. et al. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J. Clin. Invest. 118, 3588–3597 (2008). Deardorff, M.A. et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489, 313–317 (2012). Wilson, B.J., Tremblay, A.M., Deblois, G., Sylvain-Drolet, G. & Giguère, V. An acetylation switch modulates the transcriptional activity of estrogen-related receptor . Mol. Endocrinol. 24, 1349–1358 (2010). Haberland, M., Mokalled, M.H., Montgomery, R.L. & Olson, E.N. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev. 23, 1625–1630 (2009). Chang, S. et al. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell. Biol. 24, 8467–8476 (2004). Zheng, G. & Yang, Y.-C. Sumoylation and acetylation play opposite roles in the transactivation of PLAG1 and PLAGL2. J. Biol. Chem. 280, 40773–40781 (2005). Chang, S. et al. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 126, 321–334 (2006). Zhang, C.L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002). Zhang, Y. et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol. Cell. Biol. 28, 1688–1701 (2008). Song, C., Zhu, S., Wu, C. & Kang, J. Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J. Biol. Chem. 288, 28021–28033 (2013). Wang, R.-H. et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer cell 14, 312–323 (2008). Cheng, H.-L. et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl Acad. Sci. USA 100, 10794–10799 (2003). McBurney, M.W. et al. The mammalian SIR2 protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 23, 38–54 (2003). Bao, X. et al. Identification of 'erasers' for lysine crotonylated histone marks using a chemical proteomics approach. eLife 3, e02999 (2014). Eskandarian, H.A. et al. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science 341, 1238858 (2013). Rothgiesser, K.M., Erener, S., Waibel, S., Lüscher, B. & Hottiger, M.O. SIRT2 regulates NF-κB dependent gene expression through deacetylation of p65 Lys310. J. Cell Sci. 123, 4251–4258 (2010). Vaquero, A. et al. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 20, 1256–1261 (2006). 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. Bobrowska, A., Donmez, G., Weiss, A., Guarente, L. & Bates, G. SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo. PLoS ONE 7, e34805 (2012). Jin, L. et al. Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3. Protein Sci. 18, 514–525 (2009). Ahn, B.H. et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl Acad. Sci. USA 105, 14447–14452 (2008). Haigis, M.C. et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic cells. Cell 126, 941–954 (2006). Laurent, G. et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell 50, 686–698 (2013). Mathias, R.A. et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159, 1615–1625 (2014). Du, J. et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011). Nakagawa, T., Lomb, D.J., Haigis, M.C. & Guarente, L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 (2009). Tan, M. et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 19, 605–617 (2014). Xiao, C. et al. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J. Biol. Chem. 285, 36776– 36784 (2010). Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006). Jiang, H. et al. SIRT6 regulates TNF- secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496, 110–113 (2013). Vakhrusheva, O. et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 102, 703– 710 (2008). Ryu, D. et al. A SIRT7-Dependent acetylation switch of GABP1 controls mitochondrial function. Cell Metab. 20, 856–869(2014). Akeson, E.C. et al. Chromosomal inversion discovered in C3H/HeJ mice. Genomics 87, 311–313 (2006).