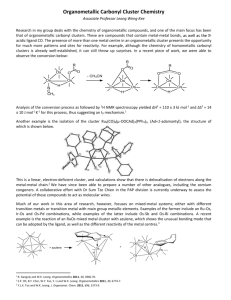
Chemistry 6210/4203: Organometallic Chemistry Instructors: Prof
advertisement

Chemistry 6210/4203: Organometallic Chemistry Instructors: Prof. Chris Kozak (ckozak@mun.ca) office C2018, lab C5006 Prof. Fran Kerton (fkerton@mun.ca) office C4007, lab C5006 Course URL: http://www.chem.mun.ca/homes/cmkhome/Chem6210.html It is also linked from our departmental webpage. Class times: Monday, Wednesday and Thursday, 4:00 to 4:50 pm Location: Room C2010 Texts for 6210: “The Organometallic Chemistry of the Transition Metals”, 4th Edition by Robert H. Crabtree. Publisher: Wiley. Available through the University Bookstore or Amazon.ca. Texts for 4203: “Organometallics 1 and 2” by Manfred Bochmann. Publisher: Oxford University Press. Available through the University Bookstore or Amazon.ca. Other Texts Available in the Library: QD 172 T6 C73 2001 “The organometallic chemistry of the transition metals” 3rd ed. Crabtree, Robert H. QD 411 S65 1997 “Organometallic chemistry” Spessard, Gary O. QD 411 O73 1994 “Organometallic reagents in organic synthesis” Bateson, J. H. QD 411 M397 1991 “Organometallic chemistry: a unified approach” Mehrotra, Ram C. QD 502 J67 1991 “Reaction mechanisms of inorganic and organometallic systems” Jordan, Robert B. QD 411 E4413 2006 “Organometallics” 3rd ed. Elschenbroich, Christophe. QD 262 O745 2002 “Organometallics in synthesis: a manual” 2nd ed. Schlosser, M. In general, QD 411 is the call number for books on organometallic chemistry related topics. Encyclopedic Sources: [Electronic Resources available through the library site] “Comprehensive Organometallic Chemistry III” “Encyclopedia of Inorganic Chemistry” (Very highly recommended, especially the Chapter on Zr and Hf) Supplementary Material: Additional material will be provided in class or will be obtained from the primary literature (journal articles and communications). For example, polymerization catalyst design will be given as a hand-out in class. What is Organometallic Chemistry? An organometallic molecule is one that contains a metal-carbon bond. Organometallic chemistry is typically thought of as a subset of Inorganic/Coordination chemistry, although it also plays a major role in Organic and Materials/Polymer chemistries. Organometallics also play smaller but important roles in other branches of chemistry such as Biological and Analytical. Chemists sometimes refer to particular molecules as organometallic even though these molecules lack the required metal-carbon bond. While this is formally incorrect, it is convenient shorthand when the molecule has reaction chemistry that derives from or will lead to an organometallic molecule. Here is what the leading journal of the field, Organometallics, has to say about the matter: An "organometallic" compound will be defined as one in which there is a bonding interaction (ionic or covalent, localized or delocalized) between one or more carbon atoms of an organic group or molecule and a main group, transition, lanthanide, or actinide metal atom (or atoms). Following longstanding tradition, organic derivatives of the metalloids (boron, silicon, germanium, arsenic, and tellurium) will be included in this definition. Furthermore, research dealing with metal-containing compounds which do not contain metal1 carbon bonds will be considered as well if there is a close relationship between the subject matter and the principles and practice of organometallic chemistry. Such compounds may include, inter alia, representatives from the following classes: molecular metal hydrides; metal alkoxides, thiolates, amides, and phosphides; metal complexes containing organo-group 15 and 16 ligands; metal nitrosyls". This course will cover the structure and properties (chemical and physical) of organometallic compounds. While the course is taught from the perspective of an inorganic chemist, it will certainly find applicability for organic chemists as well. We assume a background that includes courses similar to our senior undergraduate inorganic chemistry (Chem 3211), spectroscopic analysis (Chem 3500) and organic chemistry (Chem 3411) courses, with the class aimed at the level of Crabtree's textbook for graduate students. Additionally, a substantial amount of the reading will be from the current primary literature. Course Outline: 1. Introduction/Review i. Soft versus hard ligands ii. Ligand Field and MO theory iii. Trends (Oxidation states and ligand types) 2. Properties of organometallic compounds (18 electron rule, metal-metal bonding) 3. Chemical properties of different classes of organometallic compounds, including i. Metal Alkyls and Hydrides ii. Metal Carbonyls and Phosphines iii. (pi) ligands 4. Reactions of organometallics, including i. Oxidative Addition/Reductive Elimination ii. Insertion/Elimination 5. Classics of homogeneous catalysis, e.g. hydroformylation, hydrogenation, etc. 6. Applications of organometallic chemistry, including (time permitting) i. Small molecule and C-H bond activation ii. Ethylene (and other olefin) polymerization iii. Organometallic materials and polymers iv. Organic Synthesis v. Bioorganometallic Chemistry Evaluation Scheme (Note important dates in your diary!): Assignments 10% (Two at 5%, given 1 week prior to due dates of Feb 4th and Mar 4th) Class Test 20% (Covering areas 1 to 5 above, held on Mar 11th) Essay/Term Paper 30% (Topics will be assigned by lottery in January, due MONDAY Mar 30th) Oral Presentation 10% (In-class seminar of the Essay topic, held Mar 18, 19, 23, 25, 26) Final Exam 30% (4203 Exam date TBD, 6210 Proposal due WEDNESDAY April 15. See below) The Final Exam will take two forms: For undergraduate students in Chem 4203, it will be a traditional written exam scheduled during the exam period at the end of the semester. Students will be responsible for all the material presented in the course. In addition to short answer and concept based questions, a section of the exam will involve providing longer answers to questions based on choices from a list of topics presented in class during the Oral Presentations (see below). 2 For graduate students in Chem 6210, the final exam will be a research proposal following NSERC guidelines (9 pages double line spaced, Times 12 point font, plus 1 page for references). The proposal and presentation may not be on the same topic, nor may they be exactly the same as your thesis research work. Further information on the Proposal/Exam guidelines will be given in class. Your Independent Study component (Essay and Oral Presentation) consists of several parts. In addition to the written Essay and Oral Presentation, you will provide a 1-page summary and a number of questions for your classmates based on your presentation. Treat your presentation as if you were giving a tutorial. Your goal is to teach your classmates, since your presentation will provide some of the questions for the Final Exam written by the undergraduates in Chem 4203. Expectations for the Independent Study The topics for the Essay will be selected by lottery early in the semester, so you will have plenty of opportunity to complete the Independent Study part of the course early on. For the Essay component of the Independent Study, you must first present an outline (it may be in point-form) including some primary references. Profs. Kerton or Kozak may be consulted for some lead references prior to the preparation of your outline. If your outline is approved, you will then prepare a well-written, illustrated and properly referenced essay of up to 10 pages in total (Times 12 point, double spaced). The Oral Presentation should be a tutorial for the rest of the class. You are encouraged to use PowerPoint (it’s easy to cover more material quickly). If your slides are detailed, you may provide copies of them (printed no more than four slides to a page) to the class. Otherwise, provide good quality handouts or copies of articles as appropriate (the quality of your supporting materials will be included in your assessment). Your slides should be clear, well illustrated, referenced and informative. Ideally, someone should learn a lot about your topic just by reading your slides. Remember, they will be used as study notes for the exam. The Independent Study topics will be selected from the following: Dinitrogen activation Activation of C-F bonds C-C coupling reactions Organometallic polymers Carbon dioxide as a C1 feedstock Surface organometallic chemistry Organoactinide complexes Hydrosilation catalyzed by transition metal complexes Stabilization of 15e- and 17e- organometallic radicals Ring-opening metathesis polymerizations Ring-closing metathesis reactions Catalytic asymmetric hydrogenation Homogeneous catalysis in supercritical fluids Mass spectrometry techniques in organometallics Obviously, one could write a book on any one of these topics, and indeed many have already been written. That said; do not be tempted to use a book as your main reference. Your sources should be from the primary literature (i.e. journal articles). Also, do not just cite references from a book or review without actually reading the reference itself. A book or review article should really only be used to help you identify the leading researchers or problems in your subject area. Your goal should be to focus on one aspect (a particular researcher, reaction, substrate, metal, etc.) with good depth, but also to give sufficient background so that your classmates will understand the area. 3