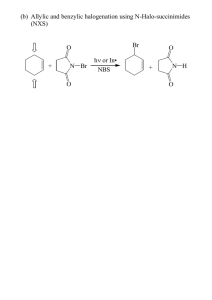
NAME Chem 204 Spring, 1990 Final Exam FINAL EXAM: Chaps. 1
advertisement
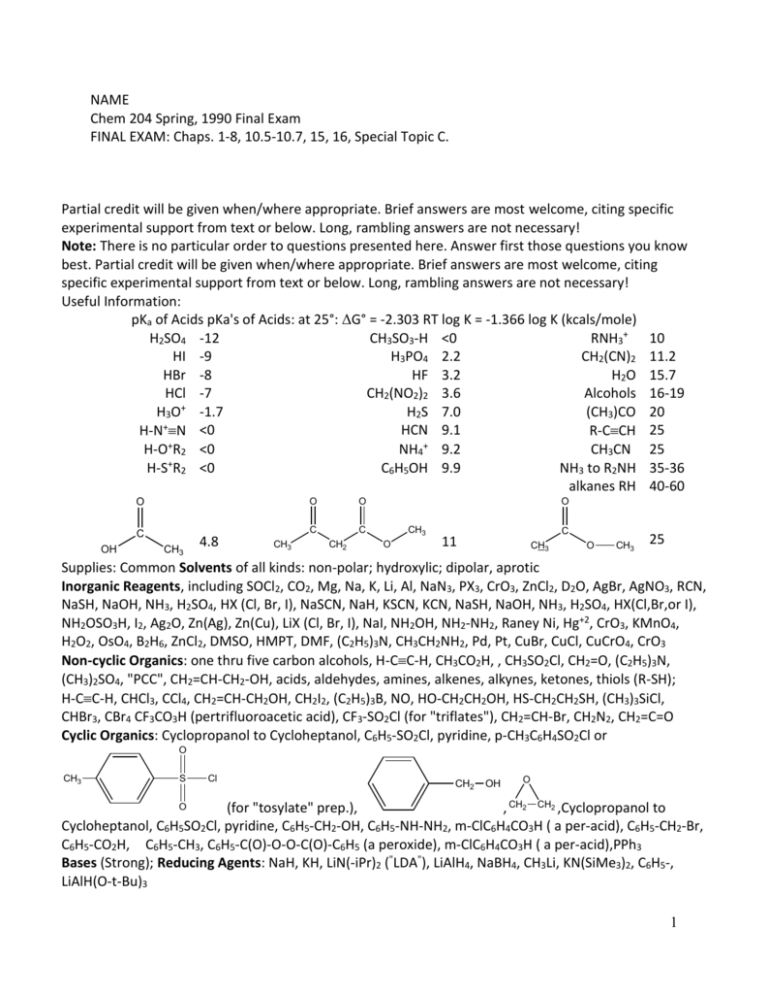
NAME Chem 204 Spring, 1990 Final Exam FINAL EXAM: Chaps. 1-8, 10.5-10.7, 15, 16, Special Topic C. Partial credit will be given when/where appropriate. Brief answers are most welcome, citing specific experimental support from text or below. Long, rambling answers are not necessary! Note: There is no particular order to questions presented here. Answer first those questions you know best. Partial credit will be given when/where appropriate. Brief answers are most welcome, citing specific experimental support from text or below. Long, rambling answers are not necessary! Useful Information: pKa of Acids pKa's of Acids: at 25°: ΔG° = -2.303 RT log K = -1.366 log K (kcals/mole) H2SO4 -12 CH3SO3-H <0 RNH3+ 10 HI -9 H3PO4 2.2 CH2(CN)2 11.2 HBr -8 HF 3.2 H2O 15.7 HCl -7 CH2(NO2)2 3.6 Alcohols 16-19 H3O+ -1.7 H2S 7.0 (CH3)CO 20 + HCN 9.1 H-N N <0 R-CCH 25 + + H-O R2 <0 NH4 9.2 CH3CN 25 H-S+R2 <0 C6H5OH 9.9 NH3 to R2NH 35-36 alkanes RH 40-60 O O C OH CH3 4.8 O C CH3 O C CH2 CH3 O C 11 CH3 O CH3 25 Supplies: Common Solvents of all kinds: non-polar; hydroxylic; dipolar, aprotic Inorganic Reagents, including SOCl2, CO2, Mg, Na, K, Li, Al, NaN3, PX3, CrO3, ZnCl2, D2O, AgBr, AgNO3, RCN, NaSH, NaOH, NH3, H2SO4, HX (Cl, Br, I), NaSCN, NaH, KSCN, KCN, NaSH, NaOH, NH3, H2SO4, HX(Cl,Br,or I), NH2OSO3H, I2, Ag2O, Zn(Ag), Zn(Cu), LiX (Cl, Br, I), NaI, NH2OH, NH2-NH2, Raney Ni, Hg+2, CrO3, KMnO4, H2O2, OsO4, B2H6, ZnCl2, DMSO, HMPT, DMF, (C2H5)3N, CH3CH2NH2, Pd, Pt, CuBr, CuCl, CuCrO4, CrO3 Non-cyclic Organics: one thru five carbon alcohols, H-CC-H, CH3CO2H, , CH3SO2Cl, CH2=O, (C2H5)3N, (CH3)2SO4, "PCC", CH2=CH-CH2-OH, acids, aldehydes, amines, alkenes, alkynes, ketones, thiols (R-SH); H-CC-H, CHCl3, CCl4, CH2=CH-CH2OH, CH2I2, (C2H5)3B, NO, HO-CH2CH2OH, HS-CH2CH2SH, (CH3)3SiCl, CHBr3, CBr4 CF3CO3H (pertrifluoroacetic acid), CF3-SO2Cl (for "triflates"), CH2=CH-Br, CH2N2, CH2=C=O Cyclic Organics: Cyclopropanol to Cycloheptanol, C6H5-SO2Cl, pyridine, p-CH3C6H4SO2Cl or O CH3 S Cl CH2 OH O (for "tosylate" prep.), , CH2 CH2 ,Cyclopropanol to Cycloheptanol, C6H5SO2Cl, pyridine, C6H5-CH2-OH, C6H5-NH-NH2, m-ClC6H4CO3H ( a per-acid), C6H5-CH2-Br, C6H5-CO2H, C6H5-CH3, C6H5-C(O)-O-O-C(O)-C6H5 (a peroxide), m-ClC6H4CO3H ( a per-acid),PPh3 Bases (Strong); Reducing Agents: NaH, KH, LiN(-iPr)2 ("LDA"), LiAlH4, NaBH4, CH3Li, KN(SiMe3)2, C6H5-, LiAlH(O-t-Bu)3 O 1 Conformational Energies for Monosubstituted Cyclohexanes Group: -ΔG° (axial – equatorial) Kcal mol-1 (25°C) F 0.25 Cl 0.5 -CN 0.2 -CCH 0.4 -OH 0.5 -CH2CH3 1.8 -OCH3 0.7 CH(CH3)3 2.1 -OTos 0.5 -OC2H5 .0.9 C6H5 3.1 -SH 0.9 -CO2CH3 1.3 Br 0.5 I 0.45 -NH2 1.0 CH3 1.7 C(CH3)3 5-6 1. (06) Whenever hydroxyaldehydes are present they often exist in the cyclic form in solution; for example (I) exists as a mixture of (II) and (III) in aqueous solutions. H OH O CH3 CH3 OH CH HO O OH- / H2O + O H OH OH H (I) (II) OH (III) H Under the conditions given, write a mechanism that accounts for the formation of (II) or (III) from (I)--your choice! 2. (12) a) Give the common or IUPAC name for (IV). O CH2 CH2 CH2 C H (IV) 2 b) (IV) can be synthesized by the use of a carboxylic acid derivative and a reducing agent, under "special" reaction conditions. Draw the pair of reagents and give the conditions to form (IV) in one operation. O CH2 CH2 CH2 C H (IV) c) Draw the reagent ("ylid"), or pair of reagents to yield an "ylid", necessary to convert (IV) into (V) in good yield--the "Wittig Reaction." O CH2 CH2 CH2 C CH2 CH2 CH2 H C H (IV) CH(CH3)2 C CH3 (V) 3.(09) Compound (VII) was required for a diterpene synthesis (JACS, 103 222(1981)). (VII) can be synthesized from (VI) but only if a "protection-deprotection" procedure, involving a temporary "mask", is incorporated into the sequence of reactions from (VI) to (VII). Outline the reactions required, in order, for a successful transformation of (VI) into (VII), including a key intermediate's structure. NOTE: Count Carbons, and Structural Chances! 3 O Br (VI) O H C OH H (VII) 4. (06) When cyclopentanthione is allowed to react with CH3ONH2 in the presence of a trace of acid catalyst (H+), it forms (IX) via the intermediate (VIII), both shown below. Outline a mechanism of several steps by which (VIII) is converted into (IX)—write the steps clearly and separately. SH 1) H+ S + CH3 O H+ N NH2 NH (VIII) O O CH3 (IX) 4 CH3 5.(10) Although we have seen routes (I) can think of two good ones for overall 100% inversion, one of one reaction and one of two reactions) for converting a chiral alcohol into its inverted alkyl chloride, there is NO one reaction that will convert a chiral alcohol into its alkyl chloride with "retained" configuration. Thus, it is necessary to use several reaction steps, each of 100% inversion or 100% retention of configuration, in order to convert (X) into (XI). Outlines below a series of such reactions by which X can be converted into XI with overall HIGH retention of configuration. In order to receive proper credit, draw the structures of any intermediate compounds, of course with their configuration clearly shown. Hint: Ask yourself, how many inversions must I have to have overall retention of configuration! CH2 HO C H CH3 (X) CH2 Cl C H CH3 (XI) 5 6.(09) Many years ago John Faulkner at Scripps described (XII), isolated from the digestive gland of the sea hare (1973). Cl CH3 CH3 Cl Br Br Br H Cl (XII) a) How many chiral (stereocenter) carbons does (XII) have? (Label each with a Star). Thus, how many stereoisomers are possible because of chiral centers (stereocenters)? b) How many bonds exhibiting both restricted rotation and geometric isomerism are in (XII)? (XII) Thus, how many geometric isomers are possible because of restricted rotation? c) What is the maximum number of stereoisomers possible due to both chirality and restricted rotation? (d) Below (XII), draw a stereoisomer that is related to (XII) as a diasteriomer. 6 7.(16) a) When CH3-S-Cl was allowed to react with (XIII), two products were obtained, (XIV) and (XV). Diagram a mechanism that accounts for the formation of (XIV) or (XV) and follow with a brief discussion as to why only two of the maximum four possible stereoisomers are found in the product mixture. Note: How is the S-Cl bond polarized? C CH3CH2 CH3 CH3CH2 H CH3SCl C CH3 Cl CH3 CH3CH2 H CH3 C C SCH3 CH3 + CH2CH2CH3 C C CH3 Cl H (XIII) (XIV) (XV) b) Consider either (XIV) or (XV). Determine the R/S designation at each stereocenter present in the stereoisomer chosen, including priorities of substituent’s at each chiral center, Note: Please clearly label as to which chiral center the R/S designation given applies. 8.(14) a) To the right of XVI, draw the two chair conformations of it, one of many halogenated 7 terpenes in red algae’s. For (b) it is essential that substituent’s be clearly shown as to whether they are axial or equatorial. OH CH3 (CH3)2CH H Br Cl CH3CH2 H (XVI) b) Which chair conformation in (a) is the more stable chair conformation(underline)? Briefly, what is the basis of your choice? Discuss. c) If (XVI) (0.1 mole) were treated with 0.1 mole of NaOCH3 in methanol, draw the structure of 1) the most likely substitution product: 2) the most likely elimination product: 9.(08) Consider again (XIII) in other reactions. Draw the structure of the organic product formed in each reaction below, including stereochemistry if that appears important. In (a), you ought to include the structure of a reaction intermediate after the first step. 8 CH3 CH3CH2 C H3O+ C CH3 H (XIII) CH3 CH3CH2 C CH3 1) O3 C 2) Zn / H2O H (XIII) 10.(10) a) Draw a dot formula(Lewis structure) for diazomethane, CH2N2, a neutral molecule in which C bears 2 H and an N, with the other N attached to the first N. Remember the octet rule! b) Calculate the formal charge on each of the atoms of your dot formula in (a), including your work to obtain these charges. c) What is the "shape" of diazomethane? What is the basis of your answer? 9 11.(05) (a) Calculate the Keq for the equilibrium, as written: H C + N R C C CN + R C C H b) Briefly, if the anion on the left side were replaced by that shown below, how would this change affect the position of the equilibrium calculated in (a)? H C + N CH(CN)2 12. (15) Consider (XVII), implicated as a mutagen in colon cancer in humans: OH HO (XVII) O =R (as a summary) a) Using Newman or flying wedge formulas, draw (and give a number to each): ALL the possible staggered conformations about the bond labeled with an arrow; the least stable eclipsed conformation about the bond labeled with an arrow: 10 b) In which conformation in (a) is the dihedral angle of the OH and OR groups = 180 decrees? c) The staggered conformations in which the -OR and -OH are gauche are each stabilized by internal (intramolecular) hydrogen bonding. For either such conformation, explain such bonding in terms of dipole and bond symbols and 5-10 words. d) Which of the two conformations mentioned in (c) is the more stable? Briefly, what is the basis of your answer? 11