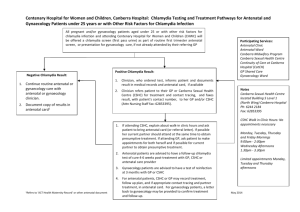
View/Open - University of Limpopo ULSpace Repository Institutional
advertisement

INTRAVENTRICULAR HAEMORRHAGE IN PREMATURE BABIES AT DR GEORGE MUKHARI HOSPITAL, PRETORIA By TIISETSO LENAKE LENTSOANE 19818782 Submitted in partial fulfilment of the requirements for the degree of MASTER OF MEDICINE (MMed) in DIAGNOSTIC RADIOLOGY AND IMAGING in the SCHOOL OF MEDICINE at the UNIVERSITY OF LIMPOPO SUPERVISORS: Prof. MPB Mawela Dr. C Minne CO-SUPERVISOR: Prof N Ebrahim 2011 TABLE OF CONTENTS DEDICATION.......................................................................................................................... IIIV DECLARATION.......................................................................................................................... V ACKNOWLEDGEMENTS ..................................................................................................... VII ABSTRACT……………………………………………………………....................................VII CHAPTER 1: BACKGROUND OF THE STUDY .................................................................... 1 1.1 1.2 1.3 1.4 1.5 1.6 INTRODUCTION .................................................................................................................... 1 THE STUDY PROBLEM ........................................................................................................... 2 RESEARCH QUESTION ........................................................................................................... 2 PURPOSE OF THE STUDY ....................................................................................................... 2 OBJECTIVES OF THE STUDY .................................................................................................. 3 SIGNIFICANCE OF PROPOSED RESEARCH ............................................................................... 3 CHAPTER 2: LITERATURE REVIEW ................................................................................... 4 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 INTRODUCTION .................................................................................................................... 4 FRAGILITY OF GERMINAL MATRIX VASCULATURE ................................................................ 5 DISTURBANCES IN THE CEREBRAL BLOOD FLOW .................................................................. 5 PLATELET AND COAGULATION DISORDERS .......................................................................... 6 THE INCIDENCE OF INTRAVENTRICULAR HAEMORRHAGE ..................................................... 6 TIMING OF HAEMORRHAGE .................................................................................................. 7 RISK FACTORS ...................................................................................................................... 7 ANTERIOR FONTANELLE VERSUS POSTERIOR FONTANELLE SCREENING................................ 9 CHAPTER 3: METHODOLOGY ............................................................................................ 10 3.1 INTRODUCTION .................................................................................................................. 10 3.2 SETTING ............................................................................................................................. 10 3.3 STUDY DESIGN ................................................................................................................... 10 3.4 STUDY POPULATION ......................................................................................................... 111 3.5 SAMPLING TECHNIQUE AND SAMPLE SIZE CALCULATION ................................................... 11 3.6 DATA COLLECTION .......................................................................................................... 122 3.7 DATA ANALYSIS ............................................................................................................... 133 3.8 RELIABILITY, VALIDITY AND OBJECTIVITY ....................................................................... 13 3.9 BIAS ................................................................................................................................... 13 3.10 ETHICAL CONSIDERATIONS .............................................................................................. 144 3.10.1 Permission ........................................................................................................... 144 3.10.2 Informed consent ................................................................................................ 144 3.10.3 Confidentiality .................................................................................................... 144 CHAPTER 4: RESULTS AND INTERPRETATION OF THE RESULTS ....................... 155 4.1 4.2 4.3 4.4 INTRODUCTION ................................................................................................................ 155 DEMOGRAPHIC INFORMATION OF THE MOTHER ................................................................ 155 DEMOGRAPHIC INFORMATION OF THE BABY .................................................................... 200 INCIDENCE OF INTRAVENTRICULAR HEMORRHAGE .......................................................... 211 i 4.5 TIMING OF INTRAVENTRICULAR HEMORRHAGE ............................................................... 222 4.6 MATERNAL RISK FACTORS FOR INTRAVENTRICULAR HEMORRHAGE ................................ 233 4.7 BABY’S RISK FACTORS FOR INTRAVENTRICULAR HEMORRHAGE ...................................... 244 CHAPTER 5: DISCUSSION OF RESULTS ......................................................................... 255 5.1 INTRODUCTION ................................................................................................................ 255 5.2 DEMOGRAPHIC INFORMATION OF THE MOTHERS .............................................................. 255 5.3 DEMOGRAPHIC INFORMATION OF THE BABIES .................................................................. 255 5.4 INCIDENCE OF INTRAVENTRICULAR HEMORRHAGE .......................................................... 266 5.5 TIMING OF INTRAVENTRICULAR HEMORRHAGE ................................................................ 266 5.6 MATERNAL RISK FACTOR FOR INTRAVENTRICULAR HEMORRHAGE .................................. 277 5.7 BABY’S RISK FACTORS FOR INTRAVENTRICULAR HEMORRHAGE ...................................... 277 5.7.1 Baby’s weight ......................................................................................................... 277 5.7.2 Gestational age ........................................................................................................ 288 5.7.3 Inotropes ................................................................................................................. 288 5.8 ANTERIOR FONTANELLE VERSUS POSTERIOR FONTANELLE SCREENING.............................. 29 CHAPTER 6: CONCLUSION AND RECOMMENDATIONS ........................................... 300 6.1 6.2 6.3 6.4 INTRODUCTION ................................................................................................................ 300 CONCLUSION ................................................................................................................... 300 LIMITATIONS OF THE STUDY ............................................................................................ 311 RECOMMENDATIONS ........................................................................................................ 311 REFERENCES ........................................................................................................................................................ 323 APPENDIX 1: DATA COLLECTION SHEET A ................................................................................................ 388 APPENDIX 2: STATISTICIAN FORM ................................................................................................................ 400 APPENDIX 3: MREC CERTIFICATE ................................................................................................................ 411 APPENDIX 4: CONSENT FORM ......................................................................................................................... 422 APPENDIX 5: INFORMATION LEAFLET ........................................................................................................ 444 APPENDIX 6: CLINICAL DATA SHEET B ....................................................................................................... 488 APPENDIX 7: HAEMORRHAGE DATA………………………………………………………………………108 LIST OF TABLES TABLE 1: RISK FACTORS.......................................................................................................................................8 TABLE 2: MATERNAL AGE ............................................................................................................................... 166 TABLE 3: ANC ........................................................................................................................................................ 177 TABLE 4: PARITY ................................................................................................................................................. 188 TABLE 5: MODE OF DELIVERY ......................................................................................................................... 19 TABLE 6: BABY’S CHARACTERISTICS, N=60 ............................................................................................... 200 TABLE 7: INCIDENCE AND SEVERITY OF IVH ............................................................................................ 211 TABLE 8: INCIDENCE AND SEVERITY OF IVH BY BABY’S WEIGHT AND GESTATIONAL AGE .. 211 TABLE 9: TIME OF BLEEDING ......................................................................................................................... 222 TABLE 10: MATERNAL RISK FACTORS FOR INTRAVENTRICULAR HEMORRHAGE ..................... 233 ii TABLE 11: BABY’S RISK FACTOR FOR INTRAVENTRICULAR HEMORRHAGE................................ 244 LIST OF FIGURES FIGURE 1: PERCENTAGE DISTRIBUTION OF MATERNAL AGE .............................................................. 16 FIGURE 2: PERCENTAGE DISTRIBUTION OF ANTENATAL CARE ATTENDANCE ........................... 167 FIGURE 3: PERCENTAGE DISTRIBUTION OF PARITY .............................................................................. 178 FIGURE 4: PERCENTAGE DISTRIBUTION OF MODE OF DELIVERY ...................................................... 19 FIGURE 5: PERCENTAGE DISTRIBUTION FOR THE TIME OF BLEEDING............................................ 22 iii DEDICATION I dedicate this dissertation to my lover and best friend, Lesiba. My love for you grows each day. Thanks for being a great husband and father, 143 always. To my lovely children, Mpho and Tshegofatso, thoughts of you sustained me and brought a smile to my face. I love you. To my parents and brother you are the best and I love you. Thanks for everything, may God continue to bless you. Above all I thank God for my lovely family and for his grace that has sustained them. iv DECLARATION I declare that INTRAVENTRICULAR HAEMORRHAGE IN PREMATURE BABIES AT DR GEORGE MUKHARI HOSPITAL, PRETORIA is my own work and that all the sources that I have used or quoted have been indicated and acknowledged by means of complete references and that this work has not been submitted before for any other degree at any other institution. TL LENTSOANE 30/09/2011 19818782 v ACKNOWLEDGEMENTS I want to thank the following persons for their respective contributions to this dissertation: My husband, Lesiba, for his unconditional love, support and encouragement. My two children, Mpho and Tshegofatso, for their support and understanding. A special thank you to my supervisors, Prof. Mawela, Dr. C Minne, for their guidance, support and encouragement. My Co-supervisor, Prof. N Ebrahim, for her support and guidance. To Prof. LDR Tsatsi for his support and encouragement. My colleague, Dr. NZ Makhanya for their willingness to participate in this study. To the Paediatric doctors for informing me about new admissions. My statistician Mr S Ntuli, for his valuable input. All mothers who gave consent for their babies to participate in the study. To the management of Dr George Mukhari Hospital for granting permission for the study. vi INTRAVENTRICULAR HAEMORRHAGE IN PREMATURE BABIES AT DR GEORGE MUKHARI HOSPITAL, PRETORIA KEYWORDS: Intraventricular hemorrhage, prematurity, hydrocephalus, gestational age ABSTRACT Background: Intraventricular hemorrhage (IVH) is a known complication occurring in the first week of life in premature neonates. The exact time of its occurrence and the ideal time to perform diagnostic imaging investigation remain controversial. Objectives: 1. To determine the incidence of intraventicular hemorrhage in premature babies at Dr George Mukhari Hospital, Pretoria. 2. To determine the timing at which bleeding occurs. 3. To determine if the rate of diagnosing intraventicular hemorrhage improves when performing ultrasound via the posterior fontanelle. 4. To determine the risk factors for intraventricular haemorrhage Materials and methods: The study included 60 premature babies of gestational age of less than 32 weeks that were admitted to our neonatal Intensive Care Unit over a two months period and screened for IVH. They were grouped into three categories according to their weight at birth, and according to their gestational age. All babies had a cranial ultrasound on day 1, 3 and 7. Results: We found that the overall incidence of IVH among premature babies was 28%. Although it did not reach statistical significance, the incidence was found to be inversely related to the birth weight and gestational age. The majority of the bleeds occurred within the first day of life and were mostly grade I and II according to Papile’s classification. The use of inotropes was found to be significantly associated with development of IVH. We also found that scanning through the posterior fontanelle did not significantly increase the rate of diagnosis for IVH. vii CHAPTER 1: BACKGROUND OF THE STUDY 1.1 Introduction Intraventricular haemorrhage (IVH) is one of the most common complications occurring in premature babies and is associated with high mortality and morbidity (Kadri, 2006:22). Sequelae of IVH include life-long neurological deficits such as cerebral palsy, developmental delay, learning disabilities, vision problems and seizures (Annibale, 2010). There are many risk factors associated with the development of IVH in neonates, prematurity being the most important risk factor. Other factors as outlined in the literature include – low Apgar scores, blood transfusion, mode of delivery, hypoxia and bicarbonate infusion. IVH is rarely seen in premature babies born after 32 weeks gestation (Thorp, 1999:11). The severity of IVH is graded from I to IV, from least to most severe according to the Papile’s classification: Grade I is limited to the germinal matrix; grade II intraventricular haemorrhage; grade III intraventricular haemorrhage with ventricular dilatation; grade IV intraparenchymal and intraventricular haemorrhage (Papile, 1978:92). The day of life on which most of the bleeding episodes occur remains controversial. Literature reveals that bleeding may occur within 24-48 hours of birth and that the majority occur during the first 4 days of life (Kadri, 2006:22). Thorp et al. (1999:11) conforms to the first few days. IVH is readily diagnosed using ultrasound. The advantages include the easy availability of ultrasound, no associated radiation and quick acquisition time. The disadvantages however is that it is operator dependant (Eber, 1991:2) IVH is associated with a high mortality and morbidity based on the severity of the haemorrhage. Short term sequelae of IVH include post-haemorrhagic hydrocephalus and periventricular leukomalacia (Ballabh, 2010:67; McCrea, 2008:35). Life-long neurological deficits include cerebral palsy, developmental delay, and seizures. The majority of the infants are asymptomatic and the diagnosis is based on screening cranial ultrasound (Ballabh, 2010:67). Early screening is very important as complications can be prevented. Cranial imaging of infants by ultrasound is routinely done via the anterior fontanelle. In a study done by Anderson et al. 1 (1994:6), it was found that scanning via the posterior fontanelle improved the chances of detecting IVH. In his study of 259 neonates, 23 had IVH diagnosed via the anterior fontanelle views and when the posterior fontanelle views where done, 11 more were diagnosed with IVH. In view of the study done by Anderson et al. (1994:6) it then becomes very important that scans be done via both fontanelles. If ultrasound imaging is done via the anterior fontanelle only, it is possible that IVH might have been missed in some of the babies. It becomes interesting to know whether the studies done by other researches where done via the anterior fontanelle only or via both fontanels. 1.2 The study problem The incidence of intraventricular haemorrhage in premature babies at Dr George Mukhari Hospital, Pretoria is unknown. Furthermore very few similar studies have been done. The incidences in studies done overseas may be of limited value in our setting due to a number of differences in the characteristics of the population, availability of resources and other risk factors associated with prematurity. This study sets out to determine the incidence of IVH in premature babies born at Dr George Mukhari Hospital. 1.3 Research question What is the incidence of intraventricular haemorrhage in premature babies born at Dr George Mukhari Hospital, Pretoria? 1.4 Purpose of the study The purpose of the study is to investigate intraventricular haemorrhage in premature babies born at Dr George Mukhari Hospital, Pretoria from October to November 2009. 2 1.5 Objectives of the study 1. To determine the incidence of intraventricular haemorrhage in premature babies at Dr George Mukhari Hospital. Pretoria. 2. To determine the time when bleeding occurs. 3. To determine if the rate of diagnosing intraventricular haemorrhage improves when performing ultrasound via the posterior fontanelle. 4. To determine risk factors for intraventricular haemorrhage. 1.6 Significance of proposed research Early diagnosis and prevention of IVH is an important component in caring for premature babies. Identification of the risk factors in our setting will enable us to be expectant of IVH and hence early intervention and screening will be instituted. Our study will indicate whether doing ultrasound through the posterior fontanelle results in improved rates of diagnosis. 3 CHAPTER 2: LITERATURE REVIEW 2.1 Introduction Intraventricular haemorrhage (IVH) is a major complication of prematurity. It typically initiates in the germinal matrix which is located on the head of the caudate nucleus and underneath the ventricular ependyma. The germinal matrix is a highly vascular collection of glial and neuronal precursor cells. The periventricular region is selectively vulnerable to haemorrhage in premature infants predominantly in the first 48hours of life. When the haemorrhage in the germinal matrix is substantial, the ependyma breaks and the ventricles fill up with blood. The majority of the infants are asymptomatic and the diagnosis is based on a screening cranial ultrasound. Some infants manifest with subtle abnormalities in the level of consciousness, movement, tone, respiration and eye movement. Uncommonly they present with decerebrate posturing, generalised tonic seizure and quadriparesis (Ballabh, 2010:67). IVH reduces the survival of premature infants and enhances the risk of neurological sequelae. A high mortality rate in premature infants is seen in those with severe IVH compared to infants without (Whitelaw, 2001:6). Premature infants with moderate to severe IVH (grade 3 – 4) are at high risk of developing post-haemorrhagic hydrocephalus cerebral palsy and mental retardation while infants with mild IVH (grade 1-2) are at risk of developmental disabilities (Murphy, 2002:87; Pinto-Martin, 1999:41). About 45-85% of premature infants with moderate to severe IVH develop major cognitive deficits and approximately 75% of these infants need special education in school (Vohr, 2003:111). Recent studies have shown better functional outcome of surviving preterm infants with periventricular haemorrhagic infarction at school age than previously thought (Roze, 2009:123). The pathogenesis of IVH is multifactorial and is commonly due to a) the inherent fragility of the germinal matrix vasculature, b) disturbance in the cerebral blood flow (CBF) and c) platelet and 4 coagulation disorders. A number of risk factors have been mentioned in the literature and induce IVH by disturbing the cerebral blood flow. Others such as thrombocytopenia contribute to IVH by causing haemostatic failure (Ballabh, 2010:67). 2.2 Fragility of germinal matrix vasculature Premature infants primarily bleed into the germinal matrix and not into the cortical mantle or white matter. This suggests that there is an intrinsic weakness in the germinal matrix vasculature compared to other parts of the brain. Endothelial tight junctions, basement membrane, pericytes and astrocyte end-feet ensheathing the blood vessels constitute the blood brain barrier. Immaturity or weakness of any of these components can cause fragility of germinal matrix vasculature (Ballabh, 2004:56; Ballabh, 2005:58; El-Khoury, 2006:59; Xu, 2008:86; Braun, 2007:27; Ballabh, 2007:13). 2.3 Disturbances in the cerebral blood flow Fluctuating CBF is associated with the development of IVH (Tsuji, 1998:44; Perlman, 1983:309; Perlman, 1985:312). Doppler technique has been used to measure CBF velocity in premature infants with respiratory distress syndrome, who are on mechanical ventilator on the first day of life. Two patterns of CBF velocity are delineated: a stable or a fluctuating CBF pattern. Stable CBF pattern consists of equal peak and trough of systolic and diastolic flow velocity, while fluctuating CBF pattern comprises of continuous alteration in systolic and diastolic flow velocity. The neonates with fluctuating CBF velocity have higher incidence of IVH compared to infants with stable CBF pattern (Perlman, 1983:309). Van Bel et al. (1987:29) reported similar findings. Other clinical conditions including hypercarbia, hypotension, patent ductus arteriosus and restlessness also contribute to the fluctuation in cerebral blood flow and hence the development of IVH (Van Bel, 1987:29; Mullaart, 1994:37; Coughtrey, 1997:47). There is disagreement on the pathogenetic role of rapid infusion of sodium bicarbonate or hyperosmolar fluids in IVH (Wigglesworth, 1976:51; Anderson, 1976:1). These factors can potentially contribute to IVH by causing episodic increase in the CBF. A rapid sodium 5 bicarbonate infusion can result in high arterial CO2 that can cause cerebral vasodilatation and hence altering cerebral hemodynamics (Ballabh, 2010:67). 2.4 Platelet and coagulation disorders A number of studies have shown that thrombocytopenia is a risk for IVH (Andrew, 1987:110; Lupton, 1988:142) however the role of coagulopathy in the pathogenesis of IVH has not been proved (Shirahata, 1990:57). 2.5 The incidence of intraventricular haemorrhage The majority of cases with IVH are mild and therefore have minimal negative prognosis. The reported incidence of IVH in the literature varies. Khodapanahandeh et al. (2007:50) reports an overall decrease in the incidence of IVH. He reports a decrease from 40% to 50% in the 1980s down to 20% to 25% in the 1990s. In a study done by Kadri et al. (2006:22) the incidence of IVH was found to be 44.68%. He studied 282 premature babies of gestation less than 37weeks admitted to neonatal ICU. He divided them into 4 subgroups, the 1st group less than 1000g, 2nd group between 1000g-1500g, 3rd group between 1500g – 2500g, 4th group greater than 2500g.Ultrasound scans were done daily in the first week, once in the second and third week. Kadri reported that the incidence was inversely related to the weight at birth. In those less than 1000g the incidence was 68.84%, in group 2 the incidence was 49.18%, group 3 had an incidence of 42.86% and 17.24% for group 4. Other studies reported an incidence of 50-62% in group 1 (Schellinger, 1988:150; Staneva, 2002:206; Trounce, 1986:61). Szymonowiz et al. (1986:22) reported a smaller figure of 49%. The incidence according to the severity of bleeding was found in the same study to be; Grade I 52.4%, Grade II 30.95%, Grade III 11.9%, and Grade IV 4.76%. Papile also reported similar results while Batton et al. (1986:3) reported 11 and 7% for Grade III and IV respectively. Leech et al. (1974:77) reported 10% for grade IV. Koksal et al. (2002:69) reported an incidence of 15%. He studied premature infants with low birth weight of 1500g and less. Fifty percent (50%) were Grade I, 17% were Grade II, 11% were 6 Grade III, and 22% were Grade IV. Seventy eight percent occurred in the first week of life. Significant risk factors and protective factors were similar to those mentioned by other studies. In a study done at Baragwanath hospital by Sandler et al. (1994:84), they reported an incidence of 53% for infants weighing less than 1500g at birth and 52% for infants born less than 35weeks gestation. Twelve percent had either Grade III or Grade IV IVH haemorrhages. The prevalence and severity if IVH was increased with both decreasing birth weight and decreasing gestational age and was also predicted by the need for active resuscitation at birth, mechanical ventilation and the development of pneumothorax. 2.6 Timing of haemorrhage Many studies showed that the haemorrhages occur during the first week of life (Chen, 1993:34; Paneth, 1993:137). The majority of the published literature agrees that IVH in premature infants does not occur immediately after birth but it occurs within 24-48hours after delivery (Jaeger, 2004:176; Tsiantos, 1974:85). Kadri (2006:22) and Thorp (1999:11) conform to bleeding occurring in the 1st few days of life. 2.7 Risk factors A number of risk factors have been proposed for the development of IVH (Table 1). 7 TABLE 1: RISK FACTORS BABIES RISK FACTORS Low gestational age and low birth-weight Gender (Controversial) REFERENCES Wells, 1995:42; Vohr, 1996:44; Heuchan, 2002:86 Oh, 1996:39; Shankaran, 1996:150; Verma, 1997:176 Gleissner, 2000:28; Weintraub, 2001:85 Shankaran, 1996:150; Gleissner, 2000:28; Synnes, 2001:138 Low Apgar Ballabh, 2010:67; Ertan, 2006:127 The presence of respiratory distress syndrome Gleissner, 2000:28; Hansen, 1999:181 Birth asphyxia Levene, 1982:57 Mechanical ventilation Cools, 1999:25; Levene, 1982:57 Hypoxia, metabolic acidosis and administration of sodium bicarbonate bolus Synnes, 2001:138; Volpe, 2008:25; Ertan, 2006:127 Anaemia and a need for transfusion Levene, 1982:57 Inotropes Lee, 2010:25 MATERNAL RISK FACTORS Mode of delivery (Controversial) Shankaran, 1996:150; Synnes, 2001:138 Hansen, 1999:181; Ment, 1995:172; Shaver, 1992:80 Maternal age, maternal infection, multiple pregnancy Levene, 1982:57 Premature rupture of membranes (PROM) Premature labour and smoking Ballabh, 2010:67 Low-dose indomethacin, caesarean section, surfactant administration, antenatal steroids, prenatal tocolytic therapy Ballabh, 2010:67 8 2.8 Anterior fontanelle versus posterior fontanelle screening It is not always stipulated in the studied literature whether the ultrasound scans were done via the anterior or posterior fontanelle. In a study done by Anderson et al. (1994:163), it is found that additional scanning via the posterior fontanelle improved the chances of detecting IVH. In his study of 259 neonates, 23 had IVH diagnosed via the anterior fontanelle views and when the posterior fontanelle views where done, additional 11 were diagnosed with IVH. In view of the study done by Anderson et al. (1994:163) it becomes important to know whether the studies done by other researchers were done via the anterior fontanelle only or via both fontanels. If they were done via the anterior fontanelle, it is possible that IVH might have been missed in some of the babies. The value of performing posterior fontanelle ultrasounds has not been determined yet. 9 CHAPTER 3: METHODOLOGY 3.1 Introduction The study methods are outlined in this chapter. These include the setting of the study, study design, study population, exclusion and inclusion criteria and sampling. The procedure for data collection and analysis, reliability, validity, bias and ethical considerations are also included in this chapter. 3.2 Setting The study was performed at Dr George Mukhari Hospital. It is a tertiary academic hospital attached to the University of Limpopo (MEDUNSA Campus). It is situated 31 kilometres north of Pretoria. It serves as a referral hospital for the surrounding clinics and hospitals from the North West, Mpumalanga and Limpopo provinces. The Neonatal unit at Dr George Mukhari hospital has fifty five beds and admits 1800 patients per annum. The majority of patients are admitted for prematurity related complications. 3.3 Study design This was a descriptive prospective study of premature babies admitted in the neonatal ICU at Dr George Mukhari Hospital. Convenience sampling was done for a period of two months (October to November 2009). Sixty babies were enrolled and studied. 10 3.4 Study population Babies with gestation less than 37weeks on admission, whose mothers were available and willing to give informed consent, were enrolled. Neonates excluded from the study were those whose mothers were not available, those whose mothers were unable to communicate with the researchers, those whose mothers were not willing to participate and those with major congenital abnormalities. 3.5 Sampling technique and sample size calculation All premature babies admitted in the ICU during the period of the study were selected to allow the largest possible sample size. The sample size of the study was calculated based on the following formula: Z 2 p (1 p ) n d2 n – sample size, which is the number of babies needed for the study, Z - confidence interval (90% ), p – proportion of babies with bleeds – estimated ( 30%), (1 – P) – those without bleed, d is the sampling error (10%). A sample size of 60 was used. 11 3.6 Data Collection Materials, apparatus and instruments The following instruments were used in the study: A high resolution sector transducer, 7. 5MHz, Mindray DC 6 machine was used. Compact Discs (CD) with the baby’s hospital number. Data sheets - Two data collection sheets were used (See Appendix 1 and 6). Data sheet A is a radiological ultrasound sheet to record all information obtained from the ultrasound scan and data sheet B is a clinical history sheet to record information about the mother and baby. From the beginning of October 2009 until the end of November 2009, 60 preterm infants with gestation of less than 37weeks admitted to the neonatal intensive care unit at Dr George Mukhari Hospital had screening ultrasounds done on day 1, day 3, and day 7 of life. Scans were done in the coronal and sagittal views via the anterior fontanelle examining the following areas: lateral ventricles on the left and right, 3rd and 4th ventricles and via the posterior fontanelle examining the occipital horns. Sonographicaly brain haemorrhages appear as localised areas of high echodensity. Images were recorded on CD and those with haemorrhages were categorized in one of the four grades according to Papile et al. (1978:92) classification. To exclude variance, all findings were checked by a consultant radiologist. The presence or absence of bleeds was later recorded on a data sheet (See Appendix 1). Infants included in the study underwent gestational age assessment that included a Ballard score on admission. The demographic and clinical data was abstracted from the patients’ medical records and the neonatal unit database. The following variables were recorded: infant- sex, birth weight, 12 gestational age, Apgar score (1 and 5 min), mode of delivery and whether the delivery was assisted or not, resuscitation at birth. The admission diagnosis was recorded with particular reference to the presence of respiratory distress syndrome, birth asphyxia, surfactant administration, hypoxia, metabolic acidosis, anaemia and a need for transfusion, thrombocytopenia, and administration of sodium bicarbonate bolus, indomethacin and inotropes. Maternal variables included: age, parity, antenatal history (See Appendix 2). 3.7 Data analysis Data for the study was entered and analysed using Microsoft Excel and EpiInfo 2002 respectively. Descriptive statistics was used to analyse data. Absolute numbers and percentages were used to interpret the data. Kruskal-Wallis test and Fisher exact test were used to determine the association between categorical variables and Mann-Whitney test for continuous variables. A p-value of less than 0.05 was considered statistically significant. 3.8 Reliability, Validity and Objectivity Reliability in this study was increased by using two readers and cases where interpretation between the readers was different were referred to a third reader whose decision was final hence validating the results of the study. The data collection form was piloted before the actual collection of data. 3.9 Bias No clinical history was available to the readers including the researcher to limit interpretive bias in the reader. One consultant from the department of Radiology at Dr George Mukhari Hospital acted as a second reader to counter the bias created by the researcher from the knowledge gained from the literature review. Readers were not allowed to do the neonatal brain ultrasound scans at the same time. 13 3.10 Ethical considerations 3.10.1 Permission Permission was sought from the superintendent of Dr George Mukhari Hospital, the Head of department of Paediatrics and Child Health, the Head of department of Diagnostic Radiology as well as the MEDUNSA Research and Ethics committee (MREC). A clearance certificate no: MREC/M/120/2009: PG was issued by MREC (See Appendix 3). 3.10.2 Informed consent Doctors in the neonatal Intensive Care Unit (ICU) were informed about the research. Informed consent was obtained by the researcher from the mothers and information leaflets translated into the local language were made available to mothers whose neonates fitted the inclusion criteria of the study (See Appendix 4 and 5). Ancillary treatment was provided when scans demonstrated bleeding. 3.10.3 Confidentiality All data was confidential. Identities did not appear on the data collection sheets or CDs used to record the ultrasound images. 14 CHAPTER 4: RESULTS AND INTERPRETATION OF THE RESULTS 4.1 Introduction In this chapter the results and the interpretation of the findings are presented. The chapter is divided into the following sections: Demographic information of the mother; Demographic information of the baby; Incidence of intraventricular hemorrhage; Association between severity of IVH and baby’s weight and gestation age; Timing of intraventricular hemorrhage; Maternal and baby’s risk factor for intraventricular hemorrhage. 4.2 Demographic information of the mother A total of 50 women who gave birth to premature babies were selected for the study. Of these women, (10) ten gave birth to twins. The median age of these women was 24 years range from 16 to 39 years. The majority (37%) of women were in the age group 20-24 years, followed by 31% aged 30 years and above (Figure 1). Thirty two (65%) women attended antenatal care (ANC) during their period of pregnancy (Figure 2). The majority (36%) of the women had two babies (Figure 3). More than fifty percent of women had normal vaginal deliveries (Figure 4). 15 TABLE 2: MATERNAL AGE Age Percentage < 20 16 20 - 24 37 25 - 29 16 > 30 31 Maternal age 40 37 35 31 percentage 30 < 20 25 20 16 16 20 - 24 15 25 - 29 10 > 30 5 0 Age Group in Years Figure 1: Percentage distribution of maternal age. 16 TABLE 3: ANC Attendance Number Percentage Booked 32 65 Unbooked 18 35 ANC 35% Booked Unbooked 65% Figure 2: Percentage distribution of ANC attendance. 17 TABLE 4: PARITY Parity Number Percentage 1 15 30 2 18 36 >3 17 34 Parity 38 36 36 Percentage 34 34 1 32 2 30 30 >3 28 26 No. of pregnancy Figure 3: Percentage distribution of parity. 18 TABLE 5: MODE OF DELIVERY Mode of delivery Percentage Caesarean section 49 Normal delivery 51 Mode of delivery 49% 51% Caesarean section Normal delivery Figure 4: Percentage distribution of mode of delivery. 19 4.3 Demographic information of the baby TABLE 6: BABY’S CHARACTERISTICS, n=60 No % Male Female Gestational age (weeks) 27 33 45 55 <30 30-33 34-36 Weight (grams) <1000 13 35 12 22 58 20 9 15 1000-1499 1500-2499 27 24 45 41 Length (cm) Skull circumference (cm) Apgar score (median, range) 1 min. 5 min. 41 (33 to 47) 29 (24 to 35) Length of Stay (days) Baby outcomes Alive Died 23 (2 to 330) Gender 6 (1 to 9) 8 (2 to 9) 52 8 87 13 Sixty premature new born babies were included in this study. Of these, 55% were female. The median gestation age of these infants was 32 weeks with a range from 26 to 37 weeks. Forty five percent of the infants weighed 1000-1499 g, 41% weighing 1500-2499 g. Only 15% (9/60) weighed less than 1000 g. The median Apgar score at one minute was 6 (range from 1 to 9) and at five minutes was 8 (range from 2 to 9) minutes. The infant length had a median of 41 cm, ranging from 33 to 47 cm, while the skull had a median circumference of 29 ranging from 24 to 35 cm. The median length of stay in the hospital was 23 days ranging from 2 to 330 days. About 20 13% of the babies died during the period of the study. Of the babies who died, 7 were alive beyond day 7 and 1 died on day 4 (Table 6). 4.4 Incidence of intraventricular hemorrhage Twenty eight percent (17/60) of new born babies had intraventricular hemorrhage (IVH) during the period of study. The majority 65% (11/17) was Grade I, 17% (3/17) were Grade II, 6% (1/17) were Grade III and 12% (2/17) were Grade IV (Table 7). TABLE 7: INCIDENCE AND SEVERITY OF IVH No 11 3 1 2 Grade I Grade II Grade III Grade IV % 65 17 6 12 TABLE 8: INCIDENCE AND SEVERITY OF IVH BY BABY’S WEIGHT AND GESTATIONAL AGE Weight <1000 g 1000-1499 g 1500-2500 g Gestational age <30 30-33 34-36 Grade I Grade II Grade III Grade IV p-value 2(20%) 4(30%) 5(50%) 2(67%) 1(33%) - 1(100%) - 2(100%) - 0.23 2(10%) 7(70%) 2(20%) 1(33%) 2(67%) - 1(100%) - 2(100%) - 0.34 p-value < 0.05 The severity of intraventricular hemorrhage was associated with baby’s weight (p=0.230) and gestation age (p=0.340) but did not reach statistical significance. Of the babies with IVH Grade I, 50% had weight between 1500-2500 grams and more than 50% had a gestation age of 30-33 weeks. The majority of bleeds were seen among infants with a birth weight of 1000-1499g and gestational age of 30-33 weeks. Only babies of gestation less than 30 weeks had Grade IV IVH. 21 4.5 Timing of intraventricular hemorrhage TABLE 9: TIME OF BLEEDING Day of bleeding Percentage 24hrs 94 72hrs 6 Bleeding 100 94 Percentage 80 60 24hrs 40 72hrs 20 0 6 Time of bleeding Figure 5: Percentage age distribution for the time of bleeding. The findings of this study revealed that, 94% (16/17) of bleeding occurred within 24 hours of admission and 6% (1/17) occurred on the third day of admission. No new bleeds were seen on day seven. Of the 16 bleeds which occurred on the first day of admission, 69% (11/16) were seen through the anterior fontanelle and 31% (5/16) were seen from both fontanels. No new bleeds were seen through the posterior fontanelle (Figure 5). 22 4.6 Maternal risk factors for intraventricular hemorrhage Maternal age was highly associated with IVH (p<0.05) and IVH was more prevalent among the younger age group. Parity and antenatal care were insignificantly associated with IVH (p>0.05). Although, 69% of the baby’s with IVH had normal vaginal delivery, mode of delivery was not significantly associated with IVH (Table 10). We also noted that only 1 pair of twins was without IVH, in the other pairs, at least one (1) had IVH. It may be difficult to associate IVH with multiple pregnancies in this study. TABLE 10: MATERNAL RISK FACTORS FOR INTRAVENTRICULAR HEMORRHAGE Age Parity ANC Booked Unbooked Mode of delivery Caesarean section Normal vaginal delivery IVH 22 (16 to 38) 2 (1 to 5) No-IVH 25 (17 to 39) 2 (1 to 5) p-value 0.041 0.099 8(53%) 7(47%) 31(63%) 18(37%) 0.117 5(31%) 11(69%) 23(53%) 20(47%) 0.077 23 4.7 Baby’s risk factors for intraventricular hemorrhage Table 11: shows the association between IVH and baby’s characteristics. No significant association was observed with regard to gestation age, gender, Apgar score, RDS, birth asphyxia, SGA, hypoxia, metabolic acidosis, anemia, blood transfusion, Bolus, IPPV, and nCPAP (p>0.05). The use of inotropes is significantly associated with bleeding (p<0.05). TABLE 11: BABY’S RISK FACTOR FOR INTRAVENTRICULAR HEMORRHAGE Gestational age IVH 31 (27 to 35) No-IVH 32 (26 to 37) p-value 0.154 Gender Male Female 6(35%) 11(65%) 21(49%) 22(51%) 0.187 Apgar score 1 min. 5 min. 7 (2 to 9) 8 (2 to 9) 6 (1 to 9) 8 (3 to 9) 0.597 0.709 RDS Birth Asphyxia SGA Hypoxia Metabolic acidosis Anemia Blood transfusion *Bolus Inotropes IPPV nCPAP 11(23%) 1(100%) 3 (14%) 10(60%) 9(60%) 8(46%) 9(53%) 11(71%) 5(33%) 4(20%) 6(40%) 35(81%) 3(30%) 4(9%) 28(64%) 24(57%) 23(55%) 26(58%) 29(65%) 3(8%) 9(21%) 23(52%) 0.514 0.364 0.472 0.501 0.548 0.405 0.488 0.465 0.028 0.611 0.301 24 CHAPTER 5: DISCUSSION OF RESULTS 5.1 Introduction IVH is the most common complication in premature infants. 20 – 25% of all premature infants will have IVH in the neonatal period. About 10 – 15% of neonates of less than 1500g birth weight have the more severe grades of hemorrhage, and over three quarters of these develop mental retardation and/or cerebral palsy in later life. (McCrea, 2008:35). 5.2 Demographic information of the mothers The median age of mothers on this study was 24 years with a range from 16 to 39 years. The majority (38%) of women were in the age group 20-24 years. Sixty six percent of women attended antenatal care during their period of pregnancy. More than fifty percent of women delivered via normal vaginal delivery. 5.3 Demographic information of the babies In our study, 53% of the babies admitted were female. The median gestation age was 32 weeks with a range from 26 to 37 weeks and the median Apgar score at first minute was 6 (range from 1 to 9) Koksal et al, (2002:69) in their study report a mean gestation of 30 weeks and the first minute Apgar score of 5. The first minute Apgar score in this study was marginally higher than in other studies. The infant length had a median of 41 cm, ranging from 33 to 47 cm, while the skull circumference had a median circumference of 29 ranging from 24 to 35 cm. The majority (43%) of the infants weighed 1000-1499g. 25 The median length of stay in the hospital was 23 days, with a range from 2 to 330 days. About 13% of the babies died during the period of the study, mostly the extremely low birth weight babies. 5.4 Incidence of intraventricular hemorrhage The reported incidence of IVH in the literature varies from 20 to 59% (Harke, 1972:37; Jaeger, 2004:176; Leech, 1974:77; Papile, 1978:92; Philip, 1989:84; Takashima, 1983:5). In our study 28% of the babies had intraventricular hemorrhage (IVH). Lee et al. (2010:25) reported an incidence of 27.8% which was similar to our study. Kadri et al. (2006:22) and Morals et al. (1986:68) in their studies reported IVH incidence of 44.68% and 43%, respectively which was higher than the one observed in our study. Our study demonstrated that the majority (65%) of IVH were Grade I, followed by Grade II (17%), 6% for grade III and 12% for grade IV. Lee et al. (2010:25) reported an incidence of 79.9% for Grade I, 6.9% for Grade II, 4.8% for Grade III and 8.6% for Grade IV which was comparable to our results. Kadri et al. (2006:22), observed (52%) Grade I and (31%) Grade II intraventricular hemorrhage among premature babies, while Koksal et al. (2002:69) reported 50% Grade I and 17% Grade II which were slightly lower compared to our results. 5.5 Timing of intraventricular hemorrhage Most studies show that hemorrhage occurs in the first few days of life. Levene et al. (1982:57) in their study report that 24% of the hemorrhages occurred in the first 24 hours of life, while 78% occurred within 72 hours. Other studies reported that 50% of bleeds occurred within the first day and 90% by the third and fourth days (Paneth, 1993:137; Partridge, 1983:102; Perlman, 1986:140). Tsiantos et al. (1974:85) reported 16% of infants he studied to have developed IVH within 24 hours, while Emerson et al. (1977:52) reported IVH to have occurred between 3 and 96 hours. In our study 94% of the bleeds occurred within 24 hours, while 6% occurred on the third day. No new bleeds were seen on day seven. 26 5.6 Maternal risk factor for intraventricular hemorrhage The results of our study indicated no significant association between intraventricular hemorrhage and mode of delivery. Although, 73% of baby’s with intraventricular hemorrhage had a normal vaginal delivery. There is no consistency in the literature on whether any mode of delivery is associated with IVH. Some studies indicate that mode of delivery has no influence on the incidence of IVH (Thorp, 1999:11; Lee, 2010:25). In contrast, Berger et al. (1997:75) reports a higher incidence of IVH after caesarean section. Koksal et al. (2002:69) in their study illustrated that caesarean section was significant protective factor against IVH. Hansen et al. (2001:181) reported similar findings. In our study, maternal age was associated with intraventricular hemorrhage; most of the babies with IVH were born to younger mothers. However Lee found no significant association between maternal age and IVH (Lee, 2010:25). 5.7 Baby’s risk factors for intraventricular hemorrhage 5.7.1 Baby’s weight In our study, there was a correlation between intraventricular hemorrhage and baby’s weight. The majority (50%) of Grade I IVH occurred in babies 1500-2500 g and 67% of Grade II had IVH occurred in babies of 1000 g or less. Khodapanahandeh et al. (2008:50) found a similar trend in his study. Koksal et al. (2002:69) reported an incidence of 56% in those with weight less than 1000g and 7% in babies with birth weight 1500 g or less. On contrary to our study the incidence of those less than 1000g was 27%. In a study done Sandler et al. (1994:84) at Baragwanath hospital IVH was found in 53% of infants weighing less than 1500g at birth, no data available for those weighing less than a 1000g and 12% were found to have either Grade III or Grade IV hemorrhages. Furthermore they reported that IVH increased with both decreasing birth weight and decreasing gestational age. 27 5.7.2 Gestational age Several studies indicated that increase in gestational age is statistically protective against IVH (Koksal, 2002:69; Berger, 1997; Khodapanahandeh, 2008:50). Similar findings were observed in this study. 5.7.3 Inotropes Our study revealed that the use of inotropes was significantly associated with IVH. Lee et al. (2010: 25) reported similar findings. No significant association was observed to IVH with regard to, gender, Apgar score, RDS, birth asphyxia, SGA, hypoxia, metabolic acidosis, and anemia, resuscitation, and the use of sodium bicarbonate infusion. Several other studies reported different findings. Khodapanahandeh et al. (2008:50) indicated that low 5- minute Apgar and RDS were statistically significant. They also reported that mechanical ventilation was significantly associated with IVH, a finding which was also found in similar studies. (Cools, 1999:80; Garcia-Prats, 1982:71). Morals et al. in his study reported that asphyxia and RDS were associated with IVH. Levene et al. (1982:57) reported that there was a strong association between RDS, IPPV, gestational age, acidosis and IVH. He also reported that RDS and severe acidosis were best predictors of IVH with IPPV and gestational age to a lesser effect. Dykes et al. (1980:66) reported that acidosis was not significantly associated with IVH which was also the case in our study. Previous studies also show that the administration of base is associated with intracranial hemorrhage (Anderson, 1976:1; Dykes, 1980:66). 28 5.8 Anterior fontanelle versus posterior fontanelle screening The majority of the reviewed literature makes no mention as to whether the cranial ultrasounds were done through both fontanels or via the anterior fontanelle only. Anderson et al. (1994:163), it was found that scanning via the posterior fontanelle improved the chances of detecting IVH. In their study of 259 neonates, 23 had IVH diagnosed via the anterior fontanelle views and when the posterior fontanelle views where done, 11 more were diagnosed with IVH. In our study the majority of the bleeds were seen through the anterior fontanelle, 5(29.4%) were seen through both fontanels. Ultrasound through the posterior fontanelle may enhance the rate of finding IVH and where feasible should be done routinely. 29 CHAPTER 6: CONCLUSION AND RECOMMENDATIONS 6.1 Introduction On the basis of the findings of the study and literature review, certain conclusions which will be outlined in this chapter were reached. Furthermore recommendations are made to the Neonatal Intensive Care Unit of Dr George Mukhari Hospital. Limitations of the study are also outlined in this chapter. 6.2 Conclusion The incidence of IVH in our study was slightly lower in comparison to other similar studies. However, in terms of the classification according to Papile, our results were comparable to other studies in general. The results indicate that lower gestational age and birth weight as well as the administration of inotropes increase the risk for IVH. Consequently prevention of prematurity would be the most effective means of treatment of IVH. During the study 8 babies whose birth weight was less than 1000 g died. The premature babies studied in the literature were bigger in comparison to our study population. The majority of patients had their intracranial bleeds within 24 hours of delivery. We can also conclude that scanning through the posterior fontanelle did not significantly increase the rate of diagnosing IVH. 30 6.3 Limitations of the Study The major limitation was the short duration of the study and hence the small sample size. The exclusion criteria also had an effect on the sample size. Some of the files could not be retrieved for analysis. 6.4 Recommendations All premature babies admitted to the neonatal ICU must have a screening cranial ultrasound within 24hours of admission. A program for prevention of prematurity must be instituted which will emphasize early identification of women at risk, education concerning causes of prematurity, early diagnosis and transfer to a perinatal center specializing in high risk deliveries. A larger follow up study on IVH in prematurity needs to be done to inform the unit protocol on screening for IVH. 31 REFERENCES 1. Kadri H, Mawla A. A, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and Intraventricular hemorrhage in preterm neonates. Childs Nerv Syst 2006; 22:1086-109. 2. Annibale DJ. Periventricular Hemorrhage-Intraventricular Hemorrhage. 2010; 3. Thorp JA, Robert D. White. Intracranial Hemorrhage in Premature Neonates: Epidemiology and Prevention. CNS Drugs 1999; 11:421-433. 4. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subepedymal and Intraventricular hemorrhage: a study of infants with birth weights less than 1500gm. J Pediatr 1978; 92(4):529-534. 5. Eber J, Villasenor C. Ultrasound: advantages, disadvantages and controversies: Nurse Pract Forum 1991 Dec; 2(4):239-242. [PubMed: 1840986] 6. Ballabh P. Intraventricular Hemorrhage in Premature Infants: Mechanism of Disease. Peadiatr Res 2010; 67(1):1-13. 7. McCrea HJ, Ment LR. The Diagnosis, Management and Postnatal Prevention of Intraventricular Hemorrhage in the Preterm Neonate. Clin Perinatol 2008; 35(4):777-vii. 8. Anderson N, Allan R, Darlow B, Malpas, Tim. Diagnosis of Intraventricular Hemorrhage in the Newborn: Value of Sonography via the Posterior fontanelle. AJR 1994; 163:893-896. 9. Whitelaw A. Intraventricular hemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol 2001; 6:135-146. [PubMed: 11483019] 10. Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed 2002; 87:F37-F41. [PubMed: 12091289] 11. Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N. Relation of cranial ultrasound abnormalities in low-birth weight infants to motor or cognitive performance at ages 2, 6 and 9 years. Dev Med Child Neurol 1994; 41:826-833. [PubMed: 10619281] 32 12. Vohr BR, Allan WC Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR. School-age outcomes of very low birth weight infants in the intraventricular hemorrhage prevention trial. Pediatrics 2003; 111:e340-e346. [PubMed: 12671149] 13. Roze E, Van Braeckel KN, van der Veere CN, Maathuis CG, Martijn A, Bos AF. Functional outcome at school age of preterm infants with Intraventricular hemorrhagic infarction. Pediatrics 2009; 123:1493-1500. [PubMed: 19482759] 14. Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Paediatr Res 2004; 56:117-124. [PubMed: 15128918] 15. Ballabh P, Hu F, Kumarasiri M, Braun A, Nedergaard M. Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex and white matter. Pediatr Res 2005; 58:791-798. [PubMed: 16189211] 16. El-Khoury N, Braun A, Hu F, Pandey M, Nedergaard M, Lagamma EF, Ballabh P. Astrocyte endfeet in germinal matrix, cerebral cortex, white matter in developing infants. Pediatr Res 2006; 59:673-679. [PubMed 16627880] 17. Xu H, Hu F, Sado Y Ninomiya Y, Borza DB, Ungvari Z, Lagamma EF, Csiszar A, Maturational changes in laminin, fibronectin, collagen IV, and perlecan in germinal matrix, cortex, and white matter and effect of betamethasone. J Neurosci Res 2008; 86:1482-1500. [PubMed : 18214989] 18. Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci 2007; 27:12012-12024. [PubMed: 17978043] 19. Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med 2007; 13:477-485. [PubMed: 17401377] 20. Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res 1998; 44:591-595. [PubMed: 9773851] 21. Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory distress syndrome. Relation to the development of Intraventricular hemorrhage. N Engl J Med 1983; 309:204-209. [PubMed 6866033] 33 22. Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med 1985; 312:1353-1357. [PubMed: 3887165] 23. Van Bel F, Van de Bor M, Stijnen T, Baan J, Ruys JH. Etiological role of cerebral blood – flow alterations in development and extension of peri-intraventricular hemorrhage. Dev Med Child Neurol 1987; 29:601-614. [PubMed: 3311857] 24. Mullaart RA, Hopman JC, Rotteveel JJ, Daniels O, Stoelinga GB, De Haan AF. Cerebral blood flow fluctuation in neonatal respiratory distress and periventricular hemorrhage. Early Hum Dev 1994; 37:179-185. [PubMed: 7925076] 25. Coughtrey H, Rennie JM, Evans DH. Variability in cerebral blood flow: observation over one minute in preterm babies. Early Hum Dev 1997; 47:63-70. [PubMed: 9118830] 26. Wigglesworth JS, Keith IH, Girling DJ, Slade SA. Hyaline membrane disease, alkali, and Intraventricular hemorrhage. Arch Dis Child 1976; 51:755-762. [PubMed: 1008580] 27. Anderson JM, Bain AD, Brown JK, Cockburn F, Forfar JO, Machin GA, Turner TL. Hyaline membrane disease, alkaline buffer treatment, and cerebral intraventicular hemorrhage. Lancet 1976; 1:117-119. [PubMed: 54636] 28. Andrew M, Castle V, Saigal S, Carter C, Kelton JG. Clinical impact of neonatal thrombocytopenia. J Pediatr 1987; 110:457-464. [PubMed: 3819949] 29. Lupton BA, Hill A, Whitefield MF, Carter CJ, Wadsworth LD, Roland EH. Reduced platelet count as a risk factor for intraventicular hemorrhage. Am J Dis Child 1988; 142:1222-1224. [PubMed: 3177331] 30. Shirahata A, Nakamura T, Shimono M, Kaneko M, Tanaka S. Blood coagulation findings and the efficacy of factor XIII concentrate in premature infants with intracranial hemorrhages. Thromb Res 1990; 57:755-763. [PubMed: 2339368] 31. Khodapanahandeh F, Khosravi N, Larijani T. Risk factors for intraventicular hemorrhage in very low birth weight infants in Tehran, Iran. Turk J Pediatr 2008; 50:247-252. 32. Schellinger D, Grant EG, Manz HJ, Patronas NJ. Intraparenchymal hemorrhage in preterm neonates: a broadening spectrum. Am J Roentgenol 1988; 150(5):1109-1115. 33. Staneva KN, Hartmann S, Uhlemann M, Dietz H, Reschke E, Koepcke E, Sadenwasser W, Kulz T. Neonatal ultrasonagraphic cerebral findings: association with risk factor for cerebral palsy. Z Geburtshilfe Neonatol 2002; 206(4):142-150. 34 34. Trounce JQ, Rutter N, Levene MI. Periventricular leukomalacia and Intraventricular hemorrhage in the preterm neonate. Arch Dis Child 1986; 61(12):1196-1202. 35. Szymonowicz W, Yu VY. Periventricular hemorrhage and leukomalacia in extremely low birth weight infants. Aust Paediatr J 1986; 22(3):207-210. 36. Batton DG, DeWitte DB, Boal DK, Nardis EE, Maisels MJ (1986) Incidence and severity of intraventricular hemorrhage: 1981-1984. Am J Perinatol. 3(4):353-356. 37. Leech RW, Kohnen P. Sub-ependymal and Intraventricular hemorrhages in the newborn. Am J Pathol 1974; 77(3):465-475. 38. Koksal N, Baytan B, Bayram Y, Nacarkucuk E. Risk factors for intraventicular hemorrhage in very low birth weight infants. Indian J pediatr 2002; 69(7):561-564. 39. Sandler DL, Cooper PA, Bolton KD, Bental RY, Simchowitz ID. Periventricularintraventricular hemorrhage in low-birth-weight at Baragwanath Hospital. S Afr Med J 1994; 84(1):26-29. 40. Chen CH, Wang TM, Wu KH, Chi CS. Intraventricular hemorrhage in preterm neonates—a two-year experience. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1993; 34(5):343348. 41. Paneth N, Pinto-Martin J, Gardiner J, Wallenstein S, Katsikiotis V, Hegyi T, Hiatt IM, Susser. Incidence and timing of germinal matrix/Intraventricular hemorrhage in low birth weight infants. Am J Epidemiol 1993; 137(11):1167-1176. 42. Jaeger M, Grussner SE, Omwandho CO, Klein K, Tinneberg HR, Klingmuller V. Cranial sonography for newborn screening: a 10-year retrospective study in 11,887 newborns. Rofo 2004; 176(6):852-858. 43. Tsiantos A, Victorin L, Relier JP, Dyer N, Sundell H, Brill AB, Stahlman M. Intracranial hemorrhage in the prematurely born infant: timing of clots and evaluation of clinical signs and symptoms. J Pediatr 1974; 85(6):854-859. 44. Wells JT, Ment LR. Prevention of Intraventricular hemorrhage in pre-term infants. Early Hum Dev 1995; 42:209-233. 45. Vohr B, Ment LR. Intraventricular hemorrhage in the preterm infant. Early Hum Dev 1996; 44:1-16. 35 46. Heuchan AM, Evans N, Henderson Smart DJ, Simpson JM. Perinatal risk factors for major Intraventricular hemorrhage in the Australian and New Zealand Neonatal Network, 19951997. Arch Dis Child Fetal Neonatal Ed 2002; 86:86-90. 47. Oh W, Fanaroff AA, Verter J, et al. Neonatal mortality and morbidity in very low birth weight (VLBW) infants: a seven year trend analysis of the Neonatal Research Network data. Pediatr Res 1996; 39:235A. 48. Shankaran S, Bauer CR, Bain R, et al. Prenatal and Perinatal risk and protective factors for neonatal intracranial hemorrhage. Arch Pediatr Adolesc Med 1996; 150:491-497. 49. Verma U, Tejani N, Klein S, et al. Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the very low-birth-weight neonate. Am J Obstet Gynecol 1997; 176:275-281. 50. Gleissner M, Jorch G, Avenerius S. Risk factors for intravetricular hemorrhage in a birth cohort of 3721 premature infants. J Perinat Med 2000; 28:104-110. 51. Weintraub Z, Solovechick M, Reichman B et al. Effect of maternal tocolysis on the incidence of severe periventricular/Intraventricular hemorrhage in very low birth weight infants. Arch Dis Child Fetal Neonatal Ed 2001; 85:F13-F17. 52. Synnes AR, Chein LY, Peliowski A et al. Variations in intraventricular hemorrhage incidence rates among Canadian neonatal intensive care units. J Pediatr 2001; 138:525-531. 53. Ertan AK, Tanriverdi HA, et al. Perinatal risk factors for neonatal intracerebral hemorrhage in preterm infants. European Journal of Obstetrics and Gynaecology and Reproductive Biology 2006; 127:29-34. 54. Hansen A, Leviton A. Labor and delivery characteristics and risks of cranial ultrasonographic abnormalities among very low birth weight infants. Am J Obstet Gynecol 1999; 181:9971006. 55. Levene MI, Fawer CL, Lamont RF. Risk factors in the development of Intraventricular hemorrhage in the preterm neonate. Arch Dis in Child 1982; 57:410-417. 56. Cools F, Offringa M Meta-analysis of elective high frequency ventilation in preterm infants with respiratory distress syndrome. Arch Dis Fetal Neonatal Ed 1999; 80:F15-F20. 57. Volpe JJ. Intraventricular hemorrhage in premature infant: current concepts part II. Ann Neurol 1989; 25:109-116. 36 58. Lee JY, Kim SK, et al. Risk factors for periventricular-intraventricular hemorrhage in premature infants. J Korean Med Sci 2010; 25(3):418-424. 59. Ment LR, Oh W, Ehrenkranz RA, et al. Antenatal steroids, delivery mode and Intraventricular hemorrhage in preterm infants. Am J Obstet Gynacol 1995; 172:795-800. 60. Shaver DC, Bada HS, Korones SB, et al. Early and late intraventricular hemorrhage: the role of obstetric factors. Obstet Gynecol 1992; 80:831-837. 61. Harke HT, Haeye RI, Storch A et al. Perinatal Cerebral hemorrhage. J Pediatr 1972; 37:80. 62. Phillip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980’s. Pediatrics 1989; 84(5):797-801. 63. Takashima S, Becker LE. Intraventricular and subependymal hemorrhage in infants dying within 10 hours of birth. Brain Dev 1983; 5(1):9-13. 64. Morals WJ, Koerten J. Obstetric management and Intraventricular hemorrhage in very-lowbirth-weight infants 1986; 68(1):35-40. 65. Partridge JC, Babcock DS, Steichen JJ, Han BK. Optimal timing for diagnostic cranial ultrasound in low birth-weight infants: detection of intracranial hemorrhage and ventricular dilation. J Pediatr 1983; 102(2):281-287. 66. Perlman JM, Volpe JJ. Intraventricular hemorrhage in extremely small premature infants. Am J Dis Child 1986; 140(11):1122-1124. 67. Emerson P, Fujimura M, Howat P, et al. Timing of Intraventricular hemorrhage. Arch Dis Child 1977; 52:183-187. 68. Berger R, Bender S, Sefkow S, Klingmuller V, Kunzel W, Jensen A. Peri/Intraventricular hemorrhage: a cranial ultrasound study on 5286 neonates. Euro J Obstet Gynecol 1997; 75:191-203. 69. Garcia-Prats JA, Porcianoy RS, Adams JM, Rudolph AJ. The hyaline membrane diseaseintraventricular hemorrhage in very low birth weight infants: perinatal aspects. Acta Paediatr Scand 1982; 71:79-84. 70. Dykes FD, Lazzara A, Ahmann P, Blumenstein B, Schwartz J, Brann AW. Intraventricular hemorrhage: a prospective evaluation on etiogenesis. Pediatrics 1980; 66:42-49. 37 APPENDIX 1: Data collection sheet A Anterior fontanelle Time Colour of the bleed Echogenic Hypoechoic Echogenic Hypoechoic Echogenic Hypoechoic Echogenic Hypoechoic Echogenic Hypoechoic Echogenic Hypoechoic Echogenic Hypoechoic Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Cysts Cysts Cysts Cysts Cysts Cysts Cysts 3 days Anterior horn Body Posterior horn Temporal horn Trigone Parafalx Other Echogenic Echogenic Echogenic Echogenic Echogenic Echogenic Echogenic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Cysts Cysts Cysts Cysts Cysts Cysts Cysts 7 days Anterior horn Body Posterior horn Temporal horn Trigone Parafalx Other Echogenic Echogenic Echogenic Echogenic Echogenic Echogenic Echogenic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Hypoechoic Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Heterogeneous Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Speckled (mixed density) Cysts Cysts Cysts Cysts Cysts Cysts Cysts 24 hour Anatomical Anterior horn Body Posterior horn Temporal horn Trigone Parafalx Other LT RT 38 APPENDIX 1: Data collection sheet A Time after delivery 24hours 3days 7days Anatomical Region Left posterior horn Right posterior horn Other Left posterior horn Right posterior horn Other Left posterior horn Right posterior horn Other Posterior fontanelle Colour of the bleed Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Echogenic Hypoechoic Heterogeneous Speckled( mixed density) Speckled( mixed density Speckled( mixed density Speckled( mixed density Speckled( mixed density Speckled( mixed density Speckled( mixed density Speckled( mixed density Speckled( mixed density Cyst Cyst Cyst Cyst Cyst Cyst Cyst Cyst Cyst 39 APPENDIX 2 STATISTICAL ANALYSES The Chairperson, Medunsa Campus Research and Ethics Committee (MCREC), Box ______________ UNIVERSITY OF LIMPOPO Medunsa Campus Dear Sir/Madam STATISTICAL ANALYSES I have studied the research protocol of DR TL LENTSOANE __________________________________________________________________________________ INTRAVENTRICULAR HEAMORRHAGE IN PREMATURE BABIES AT DR Titled: _____________________________________________________________________________ GEORGE MUKHARI HOSPITAL, PRETORIA ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ and I agree to assist with the statistical analyses. Yours sincerely, ______________________________________ Signature: Statistician SAM NTULI _______________________________________ Name in block letters 20 /07/2011 Date * Please delete which is not applicable. If you do not agree to assist with the statistical analyses, please provide reasons on a separate sheet. 40 APPENDIX 3 41 APPENDIX 4 UNIVERSITY OF LIMPOPO (Medunsa Campus) ENGLISH CONSENT FORM Statement concerning participation in a Clinical Trial/Research Project*. Name of Project / Study / Trial* ........................................................................................................................................................................... ……. ................................................................................................................................................................................... ................................................................................................................................................................................... I have read the information on */heard the aims and objectives of* the proposed study and was provided the opportunity to ask questions and given adequate time to rethink the issue. The aim and objectives of the study are sufficiently clear to me. I have not been pressurized to participate in any way. I understand that participation in this Clinical Trial / Study / Project* is completely voluntary and that I may withdraw from it at any time and without supplying reasons. This will have no influence on the regular treatment that holds for my condition neither will it influence the care that I receive from my regular doctor. I know that this Trial / Study / Project* has been approved by the Medunsa Campus Research and Ethics (MREC), University of Limpopo (Medunsa Campus) / Dr George Mukhari Hospital. I am fully aware that the results of this results of this Trial / Study / Project* will be used for scientific purposes and may be published. I agree to this, provided my privacy is guaranteed. I hereby give consent to participate in this Trial / Study / Project*. ............................................................ Name of patient/volunteer ........................................................ Signature of patient or guardian. ................................ .................................... ................................................ Place. Date. Witness ___________________________________________________________________________ Statement by the Researcher I provided verbal and/or written* information regarding this Trial / Study / Project* I agree to answer any future questions concerning the Trial / Study / Project* as best as I am able. I will adhere to the approved protocol. ....................................... .................................... Name of Researcher Signature *Delete whatever is not applicable. ...............…… Date Place 42 APPENDIX 4 UNIVERSITY OF LIMPOPO (Medunsa Campus) SETSWANA CONSENT FORM Seteitemente se se ka ga go tsaya karolo mo Tekopatlisisong / Porojeke ya Patlisiso*. Leina la Porojeke / Patlisiso / Tekelelo* ........................................................................................................................................................................... ……. ................................................................................................................................................................................... ................................................................................................................................................................................... Ke buisitse tshedimosetso mo */ke utlwile maitlhomo le maikemisetso a* patlisiso e e tshitshintsweng mme ke filwe tšhono ya go botsa dipotso le go fiwa nako e e lekaneng ya go akanya gape ka ntlha e. Maitlhomo le maikemisetso a patlisiso e a tlhaloganyega sentle. Ga ke a patelediwa ke ope ka tsela epe go tsaya karolo. Ke tlhaloganya gore go tsaya karolo mo Tekopatlisisong e / Patlisiso / Porojeke* ke boithaopo le gore nka ikgogela morago mo go yona ka nako nngwe le nngwe kwa ntle ga go neela mabaka. Se ga se kitla se nna le seabe sepe mo kalafong ya me ya go le gale ya bolwetsi jo ke nang le jona e bile ga se kitla se nna le tlhotlheletso epe mo tlhokomelong e ke e amogelang mo ngakeng ya me ya go le gale. Ke a itse gore Tekopatlisiso / Patlisiso / Porojeke* e e rebotswe ke Patlisiso le Molao wa Maitsholo tsa Khampase ya Medunsa (MREC), Yunibesithi ya Limpopo (Khampase ya Medunsa) / Bookelo jwa Ngaka George Mukhari. Ke itse ka botlalo gore dipholo tsa Tekelelo / Patlisiso / Porojeke* di tla dirisetswa mabaka a saentifiki e bile di ka nna tsa phasaladiwa. Ke dumelana le seno, fa fela go netefadiwa gore se e tla nna khupamarama. Fano ke neela tumelelo ya go tsaya karolo mo Tekelelong / Patlisiso / Porojeke* e. ............................................................ Leina ka molwetse/moithaopi ........................................................ Tshaeno ya molwetse kgotsa motlamedi. ................................ .................................... ................................................ Lefelo. Letlha. Paki ___________________________________________________________________________ Seteitemente ka Mmatlisisi Ke tlametse tshedimosetso ka molomo le/kgotsa e e kwadilweng malebana le Tekelelo / Patlisiso / Porojeke* e. Ke dumela go araba dipotso dingwe le dingwe mo nakong e e tlang tse di amanang le Tekelelo / Patlisiso / Porojeke* ka moo nka kgonang ka teng. Ke tla tshegetsa porotokolo e e rebotsweng. ....................................... .................................... ...............…… Leina la Mmatlisisi Tshaeno Letlha Lefelo *Phimola sengwe le sengwe se se seng maleba. 43 APPENDIX 5 PARTICIPANT INFORMATION AND INFORMED CONSENT INTRAVENTRICULAR HEMORRHAGE IN PREMATURE BABIES AT DR GEORGE MUKHARI HOSPITAL, PRETORIA PARTICIPANT INFORMATION INTRODUCTION I invite you and your baby to volunteer for a research study. This information sheet is to help you decide if you would like to participate. Before you agree to take part in this study you should fully understand what is involved. If you have any questions, which are not fully explained in this leaflet, please ask the interviewer. You should not agree to take part unless you are completely happy about the study. THE PURPOSE OF THIS STUDY Your baby has been born before the expected due date and may develop a bleed in the brain. We will be taking pictures of the brain using a machine that will not cause any harm but may be a little uncomfortable. This will help us to identify the bleed early and prevent complications. WHAT DOES THE STUDY INVOLVE? If you agree for your baby to take part, you will be one of approximately 60 mothers who gave birth to a premature baby. The following will be done: Your baby’s file will be studied and all problems encountered while being admitted, procedures done and medication given will be written down on a recording sheet. 44 This information will help in identifying the predisposing factors. Ultrasound scans will be performed on all premature babies within 24hours, on day 3 and on day 7 to identify any bleeding. Advantages of ultrasound scans: it is not harmful, no radiation, no side effects, easily available, the investigation will be done at the bed side in the ward. Your doctor will be informed of the results and should you wish to know the results these will be given to you. ETHICAL APPROVAL Approval has been obtained from MREC, clearance certificate no………………. YOUR RIGHTS AS A PARTICIPANT Your baby’s participation in this study is entirely voluntary and you can refuse for your baby to participate or stop at any time without stating any reason. The treatment your child obtains will not be affected at all if you decide not to participate or if you want to stop participating at any time. CONFIDENTIALITY All information obtained during this study is strictly confidential. Identifies will not appear on the data collection sheet however the identities of the babies must be known so that appropriate treatment may be delivered as part of the standard of care. Any concerns or questions can be directed to Dr TL. Lentsoane, Department of Diagnostic Radiology, MEDUNSA. Tel: 0827466779 Email: lenake@webmail.co.za Postal: Box 2417 Medunsa 0204 45 APPENDIX 5 TSHEDIMOSETSO YA MOTSAYAKAROLO LE TUMELELO E E IKAEGILENG KA TSHEDIMOSETSO TIRAGALO YA GO DUTLA MADI MO TENG GA BENTERIKELE MO MASEENG A A TSHOLWANG PELE GA NAKO KWA BOOKELONG JWA GEORGE MUHARI, PRETORIA TSHEDIMOSETSO YA MOTSAYAKAROLO MATSENO Ke laletsa wena le ngwana wag ago go ithaopa go nna karolo ya patlisiso. Tsebe e ya tshedimosetso e go thusa gore e tseye tshwetso ya gore a o tla rata ge ngwana a tsaya karolo. Pele ga o dumela gore ngwana a tsaye karolo o tshwanetse go tlhaloganya ka botlalo gore seno ke ka ga eng. Fa o na le dipotso dingwe, tse di sa tlhalosiwang ka botlalo mo tsebeng e, ke kopa o botse motho yo o go botsang dipotso. Ga o a tshwanela go tsaya karalo ntle le fa o itumelela patlisiso e ka gotlhe. MAIKEMISETSO A ITHUTO E Lesea la gago le tshotswe pele ga nako e go neng go solofetswe la ka tsholwa ka yona, ka jalo go nale kgonagalo ya gore a katswe a na le madi mo booking jwa gagwe. Re tsile go dirisa motshini oo ka se mo ameng ka gope, go tsaya ditshwantsho tsa booko jwa gagwe, fela a ka nne a se nnisege mo go ona. ITHUTO E KA GA ENG? Fa o tsaya tshwetso gore ngwana a tsaye karalo o tla nna mongwe wa bomme b aba lekanyetswang go 60 ba ba belegeng bana pele ga nako. 46 Go tla dirwa tse di lateng: Faele ya ngwana wag ago e tla ithutiwa, mathata otlhe a gagwe a tla kwala mo go yona ka nako e a tla beng a rabadiwa mo bookelong , sengwe se se tla diriwang mo go ena, le meleme yotlhe e a tla e newang, di tla kwalwa mo letlakaleng lele matshwanedi. Se, se tla re thusa mo go go itsing gore (matsapa di a tsaya kae) ke mabaka mafeng aa ka bong a na le maikarabelo a a tsisang madi mo bookong. Go leba setshwantsho sa mo teng ga karolo nngwe ya mmele go tla dirwa mo baneng botlhe b aba tshotsweng pele ga nako mo diureng tse 24, ka letsatsi la bo 3, le ka letsatsi la 7 go lemoga fa go na le madi mangwe a a dutlang. Mesola ya go leba setshwantsho sa mo teng ga karolo nngwe ya mmele: ga go kotsi, ga go na phasalalagote, ga go na ditlamorago, go fitlhelelwa bonolo, bana ba tla direlwa mo dikubikeleng tsa bona. TETLELELO GO YA KA NGWAO Tetlelelo ya go dira se, re e abetswe ke ba MREC, clearance no………….. DITSHWANELO TSA GAGO JAAKA MOTSAYAKAROLO Go tsaya karolo ga ngwana wag ago mo ithutong e ke ka go ithaopa mme a ka nna wa gana gore ngwana a tsaye karolo kgotsa wa emisa nako nngwe le nngwe kwa ntle ga go tlhalosa mabaka. Kalafi e ngwana wagago a e amogelang ga e kitla e amega ka gope fa o tsaya tshwetso ya go se tseye karolo kgotsa fa o batla go emisa go tsaya karolo ka nako nngwe le nngwe. KHUPAMARAMA Tshedimosetso e e amogetsweng ka nako ya ithuto e ke khupamarama. Ditshupo tsa batho ga di kitla di tlhagelela mo tsebeng ya tshedimosetso mme ditshupo tsa bana di tshwanetse go itsiwe gore ba fiwe kalafi e e maleba jaaka ya maemo a tlhokomelo. Batho ba o ka ikgolaganyang nabo: Ngaka TL. Lentsoane, Lefapha la Tupo ya Radioloji MEDUNSA, Mogala: 0827466779 47 1 APPENDIX 6: Clinical Data Sheet B Date of admission 18 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 17 yrs. Antenatal history X Parity 2 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT Unknown Unknown BABY DATA Gender Male Gestation age 28wks Birth weight Length 38cm Skull Circumference Admission diagnosis X 950 Female grams 25cm X X X RDS SGA LGA Anemia Hypoxia Surfactant therapy X IPPV Oxygen prongs nCPAP Respiratory support Management X X Blood Transfusion X Bolus Bolus repeat X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Curosurf Survanta Indomethacin Inotropes Yes No Sodabic infusion X Yes No Outcome X Died Alive 48 APPENDIX 6: Clinical Data Sheet B 2 Date of admission 18 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 17 yrs. Antenatal history X Parity 2 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT Unknown Unknown BABY DATA Gender Male Gestation age 28wks Birth weight Length 38cm Skull Circumference Admission diagnosis X X X 1000 Female grams 27cm RDS SGA LGA Anemia Hypoxia X Surfactant therapy Respiratory support X IPPV Oxygen prongs nCPAP Management X Bolus Bolus repeat Blood Transfusion X X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity/PDA X Curosurf Survanta Indomethacin Inotropes Yes No Sodabic infusion X Yes No Outcome X Died Alive 49 3 APPENDIX 6: Clinical Data Sheet B Date of admission 22 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 30 yrs. Antenatal history Parity Booked 3 X Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 6 8 BABY DATA Gender Male Gestation age 27 Birth weight Length 35cm Skull Circumference Admission diagnosis X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion Yes No Sodabic infusion Yes No Outcome X X 950 Female grams 24cm X X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity/PDA X Curosurf Survanta Indomethacin Inotropes Died Alive 50 4 APPENDIX 6: Clinical Data Sheet B Date of admission 02 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 22 yrs. Antenatal history Parity Booked 2 Gravidity X Unbooked 2 LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 8 8 BABY DATA Gender Male Gestation age 34wks Birth weight Length 38cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Blood Transfusion Bolus Bolus repeat X Sodabic infusion X 1360 Female grams 30cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Yes No Yes No Outcome X Died Alive 51 5 APPENDIX 6: Clinical Data Sheet B Date of admission 18 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 34 yrs. Antenatal history X Parity Gravidity Booked 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT 4 6 BABY DATA Gender X Male Gestation age 30wks Birth weight Length 35cm Skull Circumference Admission diagnosis X X Respiratory support X RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Female 1140 grams 29cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity/PDA Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 52 6 APPENDIX 6: Clinical Data Sheet B Date of admission 26 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 33 yrs. Antenatal history X Parity 3 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 3 7 BABY DATA Gender Male Gestation age 30wks Birth weight Length 34cm Skull Circumference Admission diagnosis X X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X 920 Female grams 26cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity X Curosurf Survanta Indomethacin Inotropes Yes No Outcome X Died Alive 53 7 APPENDIX 6: Clinical Data Sheet B Date of admission 26 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 33 yrs. Antenatal history X Parity 3 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 4 6 BABY DATA Gender Male Gestation age 29wks Birth weight Length 36cm Skull Circumference Admission diagnosis X X X Respiratory support Management X X Blood Transfusion Sodabic infusion X 920 Female grams 24cm RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Surfactant therapy Bolus Bolus repeat X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity X Curosurf Survanta Indomethacin Inotropes Yes No X Outcome X Yes No Died Alive 54 8 APPENDIX 6: Clinical Data Sheet B Date of admission 20 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 18 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Unknown Resuscitation Oxygen Bag & Mask ETT Apgar score 1 min 5 min 10 min Unknown Unknown BABY DATA Gender Male Gestation age 32 Birth weight Length 43cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion Yes No Sodabic infusion Yes No Outcome X X 1480 Female grams 29cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 55 9 APPENDIX 6: Clinical Data Sheet B Date of admission 27 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 36 yrs. Antenatal history X Parity 4 Booked Gravidity 4 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 7 8 BABY DATA Gender X Male Gestation age 28 Birth weight Length 38cm Skull Circumference Admission diagnosis X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion Yes No Sodabic infusion X Outcome X Female 880 grams 26cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity X Curosurf Survanta Indomethacin Inotropes Yes No Died Alive 56 10 APPENDIX 6: Clinical Data Sheet B Date of admission 19 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 18 yrs. Antenatal history X Parity 2 Booked Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT 4 6 BABY DATA Gender X Male Gestation age 33wks Birth weight Length 44cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP X Respiratory support Management Bolus Bolus repeat Blood Transfusion Yes No Sodabic infusion X Yes No X Died Alive Outcome Female 2260 grams 34cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes 57 11 APPENDIX 6: Clinical Data Sheet B Date of admission 19 / dd 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 28 yrs. Antenatal history X Parity 3 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 8 9 BABY DATA Gender X Male Gestation age 33wks Birth weight Length 43cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome Female 1650 grams 31cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 58 12 APPENDIX 6: Clinical Data Sheet B Date of admission 09 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 22 yrs. Antenatal history Parity Booked 2 Gravidity X Unbooked 2 LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No X Unknown Resuscitation Oxygen Bag & Mask X ETT Apgar score 1 min 5 min 10 min 3 2 BABY DATA Gender X Male Gestation age 32wks Birth weight Length 42cm Skull Circumference Admission diagnosis grams 31cm X X Surfactant therapy X IPPV Oxygen prongs nCPAP X Blood Transfusion X Sodabic infusion 1630 RDS SGA LGA Anemia Hypoxia Respiratory support Management Female X Outcome X Bolus Bolus repeat X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity X Curosurf Survanta Indomethacin Inotropes Yes No Yes No Died Alive 59 13 APPENDIX 6: Clinical Data Sheet B Date of admission 28 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 21 yrs. Antenatal history Parity Booked 1 X Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT 2 4 BABY DATA Gender Male Gestation age 30wks Birth weight Length 41cm Skull Circumference Admission diagnosis X X X Respiratory support X Management X 1330 Female grams 28cm RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Surfactant therapy Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Died Alive X X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Curosurf Survanta Indomethacin Inotropes 60 14 APPENDIX 6: Clinical Data Sheet B Date of admission 15 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 28 yrs. Antenatal history X Parity 3 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 7 8 BABY DATA Gender X Male Gestation age 32wks Birth weight Length 41cm Skull Circumference Admission diagnosis X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Female 1510 grams 30cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity X Curosurf Survanta Indomethacin Inotropes Died Alive 61 15 APPENDIX 6: Clinical Data Sheet B Date of admission 11 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 39 yrs. Antenatal history X Parity 4 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 4 6 BABY DATA Gender Male Gestation age 33wks Birth weight Length 42cm Skull Circumference Admission diagnosis X X Respiratory support X grams 34cm X IPPV Oxygen prongs nCPAP Surfactant therapy X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No X 1740 Female RDS SGA LGA Anemia Hypoxia Management Outcome X X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity X Curosurf Survanta Indomethacin Inotropes Died Alive 62 16 APPENDIX 6: Clinical Data Sheet B Date of admission 11 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 39 yrs. Antenatal history X Parity 4 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 3 4 BABY DATA Gender X Male Gestation age 33wks Birth weight Length 42cm Skull Circumference Admission diagnosis X X Respiratory support Management X X Blood Transfusion X Sodabic infusion X Outcome X RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat Female 1660 grams 30cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Yes No Yes No Died Alive 63 17 APPENDIX 6: Clinical Data Sheet B Date of admission 14 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 23 yrs. Antenatal history Parity Booked 3 X Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT 7 8 BABY DATA Gender X Male Gestation age 32wks Birth weight Length 38cm Skull Circumference Admission diagnosis X X X X RDS SGA LGA Anemia Hypoxia Respiratory support X IPPV Oxygen prongs nCPAP Management X Bolus Bolus repeat Blood Transfusion Sodabic infusion Female 980 grams 26cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity/PDA X Curosurf Survanta Indomethacin Inotropes Yes No X Outcome X Yes No Died Alive 64 18 APPENDIX 6: Clinical Data Sheet B Date of admission 14 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 23 yrs. Antenatal history Parity Booked 3 X Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT 6 8 BABY DATA Gender Male Gestation age 33wks Birth weight Length 39cm Skull Circumference Admission diagnosis X X Respiratory support X RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X X 1130 Female grams 27cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 65 APPENDIX 6: Clinical Data Sheet B 19 Date of admission 11 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 25 yrs. Antenatal history Parity Booked 2 Gravidity X Unbooked 2 LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No X Unknown Resuscitation Oxygen Bag & Mask X ETT Apgar score 1 min 5 min 10 min 3 6 BABY DATA Gender X Male Gestation age 29wks Birth weight Length 38cm Skull Circumference Admission diagnosis X X X Respiratory support X grams 28cm X IPPV Oxygen prongs nCPAP Surfactant therapy X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No X 1400 RDS SGA LGA Anemia Hypoxia Management Outcome Female X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity X Curosurf Survanta Indomethacin Inotropes Died Alive 66 20 APPENDIX 6: Clinical Data Sheet B Date of admission 4 / dd 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 25 yrs. Antenatal history Parity Booked 2 X Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT Unknown Unknown BABY DATA Gender X Male Gestation age 34wks Birth weight Length 37cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion Female 1150 grams 29cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes Yes No Outcome X Died Alive 67 21 APPENDIX 6: Clinical Data Sheet B Date of admission 23 / 10 dd / 2009 mm yy MATERNAL DEMOGRAPHIC DATA Age 21 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT 8 8 BABY DATA Gender X Male Gestation age 28wks Birth weight Length 36cm Skull Circumference Admission diagnosis X X Respiratory support X RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Female 1100 grams 28cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity/PDA Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 68 22 APPENDIX 6: Clinical Data Sheet B Date of admission 14 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 28 yrs. Antenatal history X Parity 2 Booked Gravidity 4 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X C/S X Unknown ETT 7 8 BABY DATA Gender X Male Gestation age 26wks Birth weight Length 33cn Skull Circumference Admission diagnosis X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Female 900 grams 29cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity/PDA X Curosurf Survanta Indomethacin Inotropes Died Alive 69 23 APPENDIX 6: Clinical Data Sheet B Date of admission 02 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 18 yrs. Antenatal history X Parity 2 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT 6 7 BABY DATA Gender Male Gestation age 32wks Birth weight Length 37cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Sodabic infusion 1360 Female grams 28cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes Yes No X Yes No X Died Alive Outcome X 70 24 APPENDIX 6: Clinical Data Sheet B Date of admission 02 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 18 yrs. Antenatal history X Parity 2 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Unknown Oxygen Bag & Mask ETT Resuscitation X Apgar score 1 min 5 min 10 min 5 6 BABY DATA Gender Male Gestation age 31wks Birth weight Length 41cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1130 Female grams 27cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes 71 25 APPENDIX 6: Clinical Data Sheet B Date of admission 06 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 32 yrs. Antenatal history Parity Booked 4 X Gravidity 4 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 3 7 BABY DATA Gender Male Gestation age 27wks Birth weight Length 34cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion X X 810 Female grams 24cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity X Curosurf Survanta Indomethacin Inotropes Yes No Sodabic infusion X Yes No Outcome X Died Alive 72 26 APPENDIX 6: Clinical Data Sheet B Date of admission 08 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 16 yrs. Antenatal history Parity Booked 1 X Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 6 7 BABY DATA Gender Male Gestation age 33wks Birth weight Length 42cm Skull Circumference Admission diagnosis X X Respiratory support X Management Blood Transfusion RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat X Sodabic infusion 1420 Female grams 29cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Yes No X Yes No X Died Alive Outcome X 73 27 APPENDIX 6: Clinical Data Sheet B Date of admission 28 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 24 yrs. Antenatal history X Parity 2 Booked Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 6 8 BABY DATA Gender Male Gestation age 30wks Birth weight Length 39cm Skull Circumference Admission diagnosis X X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Died Alive X 800 Female grams 24cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy X Prematurity Curosurf Survanta Indomethacin Inotropes 74 28 APPENDIX 6: Clinical Data Sheet B Date of admission 18 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 20 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 7 8 BABY DATA Gender X Male Gestation age 31wks Birth weight Length 42cm Skull Circumference Admission diagnosis X X Respiratory support X Management Blood Transfusion RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat X Sodabic infusion 1360 grams 28cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Yes No X Yes No X Died Alive Outcome Female 75 29 APPENDIX 6: Clinical Data Sheet B Date of admission 19 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 21 yrs. Antenatal history Parity Booked 1 X Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 5 7 BABY DATA Gender Male Gestation age 29wks Birth weight Length 38cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1380 Female grams 29cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 76 APPENDIX 6: Clinical Data Sheet B 30 Date of admission 17 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 42 yrs. Antenatal history X Parity 3 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 9 9 BABY DATA Gender X Male Gestation age 34wks Birth weight Length 47cm Skull Circumference Admission diagnosis X X Respiratory support X Management RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome Female 2240 grams 34cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 77 APPENDIX 6: Clinical Data Sheet B 31 Date of admission 30 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 18 yrs. Antenatal history X Parity 2 Booked Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 8 8 BABY DATA Gender Male Gestation age 32wks Birth weight Length 44cm Skull Circumference Admission diagnosis X Respiratory support X Management RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1780 Female grams 34cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity X Curosurf Survanta Indomethacin Inotropes 78 32 APPENDIX 6: Clinical Data Sheet B Date of admission 20 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 22 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 6 7 BABY DATA Gender Male Gestation age 30wks Birth weight Length 44cm Skull Circumference Admission diagnosis X X X Respiratory support X Management Blood Transfusion RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat X Sodabic infusion 1370 Female grams 28cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity X Curosurf Survanta Indomethacin Inotropes Yes No X Yes No X Died Alive Outcome X 79 APPENDIX 6: Clinical Data Sheet B 33 Date of admission 16 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 27 yrs. Antenatal history Parity Booked 1 Gravidity X Unbooked 3 LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT 8 9 BABY DATA Gender X Male Gestation age 35wks Birth weight Length 44cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome Female 1710 grams 30cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 80 34 APPENDIX 6: Clinical Data Sheet B Date of admission 17 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 22 yrs. Antenatal history X Parity 2 Booked Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 8 9 BABY DATA Gender X Male Gestation age 33wks Birth weight Length 41cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome Female 1730 grams 30cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 81 35 APPENDIX 6: Clinical Data Sheet B Date of admission 28 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 38 yrs. Antenatal history X Parity 5 Booked Gravidity 4 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT Unknown Unknown BABY DATA Gender X Male Gestation age 34wks Birth weight Length 42cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome Female 1700 grams 32cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 82 36 APPENDIX 6: Clinical Data Sheet B Date of admission 28 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 38 yrs. Antenatal history X Parity 5 Booked Gravidity 4 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT Unknown Unknown BABY DATA Gender X Male Gestation age 34wks Birth weight Length 43cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome Female 2020 grams 32cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes 83 37 APPENDIX 6: Clinical Data Sheet B Date of admission 29 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 23 yrs. Antenatal history Parity Booked 2 X Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT 6 8 BABY DATA Gender Male Gestation age 32wks Birth weight Length 38cm Skull Circumference Admission diagnosis X X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X X 1390 Female grams 30cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 84 38 APPENDIX 6: Clinical Data Sheet B Date of admission 29 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 23 yrs. Antenatal history Parity Booked 2 X Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 7 8 BABY DATA Gender Male Gestation age 32wks Birth weight Length 40cm Skull Circumference Admission diagnosis X X Respiratory support RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Management X X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X X 1400 Female grams 29 X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 85 39 APPENDIX 6: Clinical Data Sheet B Date of admission 14 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 24 yrs. Antenatal history Parity Booked 2 Gravidity X Unbooked 1 LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 1 3 BABY DATA Gender Male Gestation age 32wks Birth weight Length 41cm Skull Circumference Admission diagnosis X X 1490 Female grams 28cm X X X RDS SGA LGA Anemia Hypoxia Surfactant therapy X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No X Died Alive Outcome X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Curosurf Survanta Indomethacin Inotropes 86 40 APPENDIX 6: Clinical Data Sheet B Date of admission 12 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 24 yrs. Antenatal history Parity Booked 2 Gravidity X Unbooked 1 LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT 3 4 BABY DATA Gender Male Gestation age 32wks Birth weight Length 41cm Skull Circumference Admission diagnosis X X 1310 Female grams 30cm X X X RDS SGA LGA Anemia Hypoxia Surfactant therapy X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No X Died Alive Outcome X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity X Curosurf Survanta Indomethacin Inotropes 87 41 APPENDIX 6: Clinical Data Sheet B Date of admission 03 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 18 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X Unknown ETT 9 9 BABY DATA Gender Male Gestation age 30wks Birth weight Length 43cm Skull Circumference Admission diagnosis X RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Sodabic infusion X Outcome X X 1400 Female grams 29cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes Yes No Yes No Died Alive 88 42 APPENDIX 6: Clinical Data Sheet B Date of admission 09 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 30 yrs. Antenatal history X Parity 4 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT Unknown Unknown BABY DATA Gender X Male Gestation age 33wks Birth weight Length 43cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support Management IPPV Oxygen prongs nCPAP X Blood Transfusion X Sodabic infusion X Outcome X Bolus Bolus repeat Female 1810 grams 32cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes Yes No Yes No Died Alive 89 APPENDIX 6: Clinical Data Sheet B 43 Date of admission 09 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 30 yrs. Antenatal history X Parity 4 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT Unknown Unknown BABY DATA Gender X Male Gestation age 33wks Birth weight Length 42cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion X Sodabic infusion X Outcome X Female 1540 grams 30cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Yes No Yes No Died Alive 90 44 APPENDIX 6: Clinical Data Sheet B Date of admission 09 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 39 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 9 9 BABY DATA Gender Male Gestation age 34wks Birth weight Length 43cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 2300 Female grams 32cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 91 45 APPENDIX 6: Clinical Data Sheet B Date of admission 10 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 21 yrs. Antenatal history Parity Booked 2 X Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 6 6 BABY DATA Gender X Male Gestation age 33wks Birth weight Length 37cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Female 1240 grams 27cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity/PDA X Curosurf Survanta Indomethacin Inotropes Died Alive 92 46 APPENDIX 6: Clinical Data Sheet B Date of admission 18 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 23 yrs. Antenatal history X Parity 2 Booked Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 6 7 BABY DATA Gender X Male Gestation age 27wks Birth weight Length 36cm Skull Circumference Admission diagnosis X X Respiratory support X Management RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Female 1100 grams 27cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 93 APPENDIX 6: Clinical Data Sheet B 47 Date of admission 27 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 16 yrs. Antenatal history Parity Booked 1 X Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 7 8 BABY DATA Gender Male Gestation age 32wks Birth weight Length 40cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1400 Female grams 28cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 94 APPENDIX 6: Clinical Data Sheet B 48 Date of admission 23 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 22 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X No X Unknown ETT 8 9 BABY DATA Gender Male Gestation age 31wks Birth weight Length 42cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Sodabic infusion 1690 Female grams 27cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes Yes No X Yes No X Died Alive Outcome X 95 49 APPENDIX 6: Clinical Data Sheet B Date of admission 19 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 22 yrs. Antenatal history X Parity 2 Booked Gravidity 2 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT 2 5 BABY DATA Gender X Male Gestation age 28wks Birth weight Length 37cm Skull Circumference Admission diagnosis X X X Respiratory support Surfactant therapy Blood Transfusion X Yes No X 27cm IPPV Oxygen prongs nCPAP Bolus Bolus repeat Outcome grams X X X 1140 RDS SGA LGA Anemia Hypoxia Management Sodabic infusion Female X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Curosurf Survanta Indomethacin Inotropes Yes No Died Alive 96 APPENDIX 6: Clinical Data Sheet B 50 Date of admission 13 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 37 yrs. Antenatal history X Parity 3 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 8 9 BABY DATA Gender Male Gestation age 33wks Birth weight Length 42cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support Management IPPV Oxygen prongs nCPAP X Blood Transfusion Bolus Bolus repeat X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1650 Female grams 30cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 97 APPENDIX 6: Clinical Data Sheet B 51 Date of admission 18 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 32 yrs. Antenatal history X Parity 3 Booked Gravidity 5 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 7 8 BABY DATA Gender Male Gestation age 31wks Birth weight Length 41cm Skull Circumference Admission diagnosis X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1600 Female grams 30cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity Surfactant therapy X Curosurf Survanta Indomethacin Inotropes 98 APPENDIX 6: Clinical Data Sheet B 52 Date of admission 10 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 38 yrs. Antenatal history X Parity 5 Booked Gravidity 4 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 4 8 BABY DATA Gender X Male Gestation age 33wks Birth weight Length 41cm Skull Circumference Admission diagnosis Respiratory support X X Management RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No Outcome X Female 1620 grams 30cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Prematurity/PDA Surfactant therapy X Curosurf Survanta Indomethacin Inotropes Died Alive 99 APPENDIX 6: Clinical Data Sheet B 53 Date of admission 20 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 26 yrs. Antenatal history X Parity 3 Booked Gravidity 3 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 8 9 BABY DATA Gender Male Gestation age 37wks Birth weight Length 44cm Skull Circumference Admission diagnosis X X X Respiratory support X Management RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 2280 Female grams 35cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 100 APPENDIX 6: Clinical Data Sheet B 54 Date of admission 27 dd / 09 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 27 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 6 8 BABY DATA Gender Male Gestation age 36wks Birth weight Length 45cm Skull Circumference Admission diagnosis X RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1670 Female grams 32cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 101 APPENDIX 6: Clinical Data Sheet B 55 Date of admission 11 dd / 11 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 24 yrs. Antenatal history X Parity 1 Booked Gravidity 1 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery X NVD C/S Assisted delivery Yes If yes, Vacuum Forceps Antenatal steroids Yes No Resuscitation Oxygen Bag & Mask Apgar score 1 min 5 min 10 min X No X Unknown ETT 9 9 BABY DATA Gender Male Gestation age 37wks Birth weight Length 44cm Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support Management IPPV Oxygen prongs nCPAP X Blood Transfusion Bolus Bolus repeat X Yes No X Yes No X Died Alive Sodabic infusion Outcome X 1700 Female grams 31cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Prematurity Curosurf Survanta Indomethacin Inotropes 102 APPENDIX 6: Clinical Data Sheet B 56 Date of admission 25 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 39 yrs. Antenatal history X Parity 4 Booked Gravidity 4 Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids Yes No Oxygen Bag & Mask Resuscitation X Apgar score 1 min 5 min 10 min X Unknown ETT 8 8 9 BABY DATA Gender Male Gestation age 32wks Birth weight Length 42cm Skull Circumference Admission diagnosis X X X X Respiratory support X RDS SGA LGA Anemia Hypoxia IPPV Oxygen prongs nCPAP Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No X Died Alive Outcome X 1670 Female grams 31cm Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy X Curosurf Survanta Indomethacin Inotropes 103 APPENDIX 6: Clinical Data Sheet B 57 Date of admission 26 dd / 10 / mm 2009 yy MATERNAL DEMOGRAPHIC DATA Age 34 yrs. Antenatal history Parity Booked 2 Gravidity X Unbooked 2 LABOUR AND DELIVERY DATA Mode of Delivery NVD X C/S Assisted delivery Yes X No If yes, Vacuum Forceps Antenatal steroids X Yes No Unknown Resuscitation X Oxygen Bag & Mask ETT Apgar score 1 min 5 min 10 min 3 6 7 BABY DATA Gender Male Gestation age 28wks Birth weight Length 37cm Skull Circumference Admission diagnosis X X X RDS SGA LGA Anemia Hypoxia X IPPV Oxygen prongs nCPAP Respiratory support Management X Bolus Bolus repeat Blood Transfusion X Yes No Sodabic infusion X Yes No X Died Alive Outcome X 1010 Female grams 28cm X Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy HMD X Curosurf Survanta Indomethacin Inotropes 104 APPENDIX 6: Clinical Data Sheet B 58 Date of admission / dd / mm yy MATERNAL DEMOGRAPHIC DATA Age Antenatal history yrs. Parity Booked Gravidity Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD C/S Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Unknown Resuscitation Oxygen Bag & Mask ETT Apgar score 1 min 5 min 10 min BABY DATA Gender Male Gestation age Birth weight Length Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion Yes No Sodabic infusion Yes No Outcome Died Alive Female grams Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Curosurf Survanta Indomethacin Inotropes 105 APPENDIX 6: Clinical Data Sheet B 59 Date of admission / dd / mm yy MATERNAL DEMOGRAPHIC DATA Age Antenatal history yrs. Parity Booked Gravidity Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD C/S Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Unknown Resuscitation Oxygen Bag & Mask ETT Apgar score 1 min 5 min 10 min BABY DATA Gender Male Gestation age Birth weight Length Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion Yes No Sodabic infusion Yes No Outcome Died Alive Female grams Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Curosurf Survanta Indomethacin Inotropes 106 60 APPENDIX 6: Clinical Data Sheet B Date of admission / dd / mm yy MATERNAL DEMOGRAPHIC DATA Age Antenatal history yrs. Parity Booked Gravidity Unbooked LABOUR AND DELIVERY DATA Mode of Delivery NVD C/S Assisted delivery Yes No If yes, Vacuum Forceps Antenatal steroids Yes No Unknown Resuscitation Oxygen Bag & Mask ETT Apgar score 1 min 5 min 10 min BABY DATA Gender Male Gestation age Birth weight Length Skull Circumference Admission diagnosis RDS SGA LGA Anemia Hypoxia Respiratory support IPPV Oxygen prongs nCPAP Management Bolus Bolus repeat Blood Transfusion Yes No Sodabic infusion Yes No Outcome Died Alive Female grams Birth asphyxia AGA Thrombocytopenia Metabolic acidosis Other(specify) Surfactant therapy Curosurf Survanta Indomethacin Inotropes 107 APPENDIX 7: Haemorrhage Data 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 24hour Anatomical body/post/trig/other Bleeding ant24 post24 Site Colour Cysts yes yes yes L Echo/Hetero yes no no no yes yes yes body/post/trig/other L Echo no no no yes yes no body L Echo no yes no body L yes no no no no no no no no no no yes no body L yes no no no no no no yes yes no body LR Echo no no no yes yes yes ant/body/post/tempo/trig/other LR Echo no no no no no no no no no yes yes yes body LR Echo no no no no no no no no no no no no no no no no no no no no no no no no yes yes no Other R Echo yes yes no body LR Echo yes yes no Other LR Echo no no no no no no no no no yes yes no Ant/body/other LR Echo yes yes yes no body LR echo/other yes yes no body L Echo no no no no no no yes yes no body R Echo no no no no no no 108 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 no no yes no no no no yes no yes no no no no no no no no no no yes yes no no no no yes no yes no no no no no no no no no no no no no no no no no no yes no no no no no no no no no Ant body LR LR Echo yes body LR Echo body/posterior/trigone LR Echo 109 APPENDIX 7: Haemorrhage Data 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 Bleeding yes no yes no yes no no no no no no no yes no yes no no no yes no yes no no no no no no yes yes yes no no no yes yes yes no no yes no no no no ant3 yes no yes no yes yes no no no yes no no yes no yes no no no yes no yes no no no no no no yes yes yes no no no yes yes yes no no yes no no no yes post3 yes no yes no no no no no no no no no no no yes no no no yes no no no no no no no no no no no no no no no no no no no no no no no no 3 days Anatomical body/post/trig/other Site L Colour Echo/Hetero body/post/trig/other L Echo body body L L Echo body L body LR Echo ant/body/post/tempo/trig/other LR Echo body LR Echo body R Echo Other body body/post/trigone R LR LR Echo Echo Echo other body body R L L Echo Echo Echo body R Echo Ant LR Cysts yes yes yes yes yes 110 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 yes no no no no yes no yes no no no no no no no no no yes no no no no yes no yes no no no no no no no no no no no no no no no no yes no no no no no no no no no body LR Echo body LR Echo Ant/body/posterior/trigone LR Echo 111 APPENDIX 7: Haemorrhage Data 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 Bleeding yes no yes no yes no no no no no no no yes no yes no no no yes no yes no no no no no no yes yes yes no no no yes yes yes no no yes no no no no ant7 yes no yes no yes yes no no no no no no yes no yes no no no yes no yes no no no no no no yes yes yes no no no yes yes yes no no yes no no no yes post7 yes no yes no no no no no no no no no no no yes no no no yes no no no no no no no no no no no no no no no no no no no no no no no no 7 days Anatomical body/post/trig/other Site L Colour Echo/Hetero body/post/trig/other L Echo body body L L Echo body LR Echo ant/body/post/tempo/trig/other LR Echo body LR Echo body/other LR Echo/hetero Other body ant/body/post/temporal/trigone R LR LR Echo Echo Echo other body body R L L Echo Echo Echo body R Echo Ant LR Cysts yes yes yes yes 112 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 yes no no no no yes no yes no no no no no no no no no yes no no no no yes no yes no no no no no no no no no no no no no no no no yes no no no no no no no no no body LR Echo body LR Echo temporal L Echo 113 114 60 115