Theobromine Monograph
advertisement

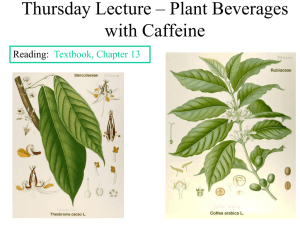
Theobromine Monograph Found in: Theobroma cacao, Camellia ptilophylla, Camellia irrawadiences, Camellia sinensis, Coffea arabica, Cola acuminata, Illex paraguariensis, and others Biosynthetic pathway: Purine of the alkaloids pathway Biosynthesis: Inosine 5'-monophosphate (IMP) or xanthosine 5'-monophosphate (XMP), derived from purine rings, undergo methylation by SAM which transfers a positive charge to glycosylated nitrogen in order to form 7-methyl XMP . Hydrolysis of the phosphate ester generates 7-methylxanthosine and further hydrolysis of the glycosidic bond produces 7methylxanthine. Subsequent methylation by SAM results in theobromine (3,7-dimethylxanthine). Medicinal/nutritional uses: Mildly improves bronchodilation (especially in asthma), possibly reduces cough reflex by suppression of vagus nerve activity and has a mild diuretic effect. Action: Smooth muscle relaxant (especially bronchial), bronchodilator, diuretic; potential action: antitussive and vasodilator Mechanism of action: Theobromine is a competitive nonselective phosphodiesterase inhibitor, which prevents the enzymatic conversion of active cAMP to the inactive form. Subsequently, cAMP levels increase which mimics the action of catecholamines leading to relaxation of bronchial smooth muscle, bronchodilation, diuresis, and vasodilation (to a lesser extent). Theobromine is also a nonselective adenosine receptor antagonist, although it has been found to be 2-3 times less active than caffeine as an adenosine A1 receptor antagonist and at least 10 times less active than caffeine as an A2 receptor antagonist. The renal vascular system is regulated by adenosine A1 and A2 receptors of which theobromine has an affinity for which may explain its diuretic nature. Theobromine Theobroma cacao Possible role in nature: Autotoxic and allelopathic effects for protection against predators Method of extraction: Cocoa seeds (‘beans’) contain 1-4% theobromine which passes from the kernel into the husk during fermentation and roasting. Commercially, theobromine is produced from cocoa husks which are first decocted with water and then filtered. The tannins are then precipitated with lead acetate and subsequently filtered. Lead is then removed and the filtrate is evaporated to an ideal level of dryness. Theobromine is extracted from the residue via alcohol and purified by recrystallization from water. References: Dewick PM. Medicinal Natural Products – A Biosynthetic Approach. 3rd ed. United Kingdom: John Wiley & Sons; 2009. Evans WC. Trease and Evans Pharmacognosy. 15th ed. Edinburgh: WB Saunders; 2002. Osbourn A, Lanzotti V. Plant-derived Natural Products: Synthesis, Function, and Application. New York: Springer-Verlag; 2009. Usmani O, Belvisi M, Patel H, Crispino N, Birrell M, Korbonits M, Korbonits Dand, Barnes P. Theobromine inhibits sensory nerve activation and cough. FASEB. 2005;19:231-233. Koyamaa Y, TomodabY, Katoa M, Ashihara H. Metabolism of purine bases, nucleosides and alkaloids in theobromine-forming Theobroma cacao leaves. Plant Physiology and Biochemistry. Nov 2003;41(11–12):977–984.