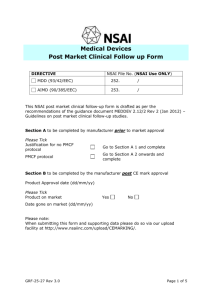
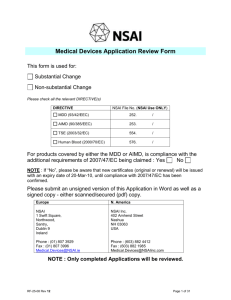
Medical Device CE Marking: Documentation Requirements
advertisement

Certification of Medical Devices (New applications and three-year review) Documentation Requirements for Manufacturers For CE Marking of medical devices, NSAI is mandated to perform a comprehensive review of product information held by your company in compliance to the relevant Directive. Based upon a successful review, NSAI will issue your product certificates for a three years period. At the end of this 3-year you are required to apply for re-certification. The following is a comprehensive list of the documentation, which must be supplied in English, in support of your application, either a new application, a transfer from another Notified Body or a Three-year review. Customer supplied information in support of Product Reviews (new applications, threeyear reviews and transfers from other Notified Bodies): o A completed/populated application form Under EU directive 93/42/EEC, 98/79/EC, Type of Medical Devices For Medical Devices, Active Implantable Medical Devices, 90/385/EEC 2003/32/EC In Vitro Diagnostic Medical Devices For Medical Devices manufactured utilising tissue of animal origin For Medical Devices incorporating stable derivatives of human blood and human plasma For Medical Devices with medicinal products 2000/70/EC 2001/83/EC Use form GRF-25-16, (new applications) GRF-25-30, (3 year review applications) GRF-25-26 GRF-25-16 & Appendix A GRF-25-16 & Appendix B GRF-25-16 & Appendix C Please submit two copies of the application- one in Word ™ format and one as a signed controlled copy (i.e. pdf). This form is to be submitted electronically to medical.devices@nsai.ie or medical.devices@nsaiinc.com For new applications and product transfers, please provide a sample of the device or CD Rom images of performing devices. All supporting documentation to be supplied to NSAI prior to the scheduled date. Description of Device Provide a description with as much detail about the device as possible. Intended Use of the device Be specific and concise. This information is vital in verifying the correct product classification and falls under the scope of the relevant directive. Existing Device Approvals For example FDA 510(k) or PMA, Canadian License, CE marking etc. Classification of Device & Classification Rule GAD-25-22 Rev 5.0 1 Certification of Medical Devices (New applications and three-year review) Documentation Requirements for Manufacturers Identify the classification of your medical device and the applicable classification rule and provide a rationale for your decision. For Medical Devices, use guidance document MEDDEV 2.4/1 Product Labelling Provide copies of sample labelling and user information supplied with the device, demonstrating compliance to the Essential Requirements. Ensure that all the risks identified in the risk analysis are adequately mitigated in the labelling provided. Essential Requirements (Annex I) / Use of Harmonized Standards An Essential Requirements checklist, demonstrating compliance to current relevant harmonized standards shall be provided including date of issue of the standard. The checklist shall clearly identify whether or not each essential requirement is applicable to your device. If an essential requirement is applicable, the company must demonstrate how this requirement was met. For example, compliance with a relevant harmonized standard is presumed to demonstrate compliance with the requirement. If a relevant harmonized standard is available but not followed, the company must provide a rationale. Risk Management: Provide objective evidence of compliance with the harmonised standard for risk management – EN ISO 14971:2012 An up-to-date Risk Analysis of the device, including a risk-to-benefit conclusion shall be provided. The risk-to-benefit conclusion statement must be signed and dated by the responsible individual(s). For 3-year reviews, the updated Risk Analysis shall include information gained from the post-production phase and customer feedback. Sterilization (if applicable) For new applications, provide a copy of the protocol and report for the most recent sterilisation revalidation. NSAI reserves the right to request a copy of the original validation. For 3-year reviews, provide a copy of the protocol and report of the most recent revalidation. All revalidations should show compliance with relevant EN Harmonized standards for sterilization (e.g. EN ISO 11135, EN ISO 11137, etc) Biocompatibility Provide evidence that all patient contact components of the device are biocompatible, through compliance to EN ISO 10993 standard series. Product Shelf Life / Package Stability Provide objective evidence of the stability of the device for the stated shelf life of the device, through both accelerated and real-time data. If the device is sterile, compliance to the packaging standard EN ISO 11607-2009 is required. Other required standards may apply depending upon the device. For IVDs, provide data which supports the performance of the product (with respect to population, specificity and sensitivity etc). GAD-25-22 Rev 5.0 2 Certification of Medical Devices (New applications and three-year review) Documentation Requirements for Manufacturers Clinical Evaluation / Clinical data Clinical data must be provided for all medical devices as outlined below: For Class I (Sterile or Measuring) and Class IIA medical devices, clinical literature relevant to the specific device and its intended use must be provided, where equivalency can be demonstrated. For Class IIB and Class III high-risk medical devices, either the results of specific clinical investigations or a combination of specific clinical investigations, plus a critical evaluation of relevant clinical literature for the device is required, where equivalency can be demonstrated. This critical evaluation must be provided by a relevant clinical expert and must substantiate the equivalency of the clinical literature to the medical device under review. In cases where plans for a clinical investigation have been submitted to a Competent Authority, please supply a copy of this plan to the NSAI Note: For Class 1 Sterile and Measuring devices NSAI is only required to review the details pertaining to the sterility or measuring of the device. Note: requirements for clinical evaluation/clinical data will be revised with the updated Medical Devices Directive Compliance with EU Harmonized Standards: A statement of compliance to the FDA requirements or the ANSI/AAMI standards is not sufficient. If a relevant EU harmonized standard is available but not followed, the company shall provide a rationale. For Harmonised Standards listing, see http://ec.europa.eu/enterprise/newapproach/standardization/harmstds/reflist.html Subcontracted Services Provide a copy of all valid QMS certificates for subcontracted service providers so as to verify adequacy of associated scope. Where applicable, a copy of the valid EC Type certificate must be submitted. The subject device shall be covered by the subcontractor’s QMS certificate scope. For Class III products (Annex II only) Provide data to demonstrate that design outputs met the input requirement for design and development. Also show that design validation demonstrates that the product is capable of meeting the requirements for its intended use. Provide manufacturing information, detailing all aspects of the manufacturing process. Transfer of certification For transfer of product certification from another Notified Body, in addition please submit the following: Transition plan, including proposed end dates Copy of existing certification Permission to contact existing Notified Body GAD-25-22 Rev 5.0 3 Certification of Medical Devices (New applications and three-year review) Documentation Requirements for Manufacturers Three-Year Review For continued CE Marking of medical devices, NSAI is mandated to perform a comprehensive review of product certifications held by your company on a three-year cycle. Please understand that several of the items requested below as part of the review process are as a direct result of the EU harmonisation and implementation of new or revised medical device standards, additional technical experience gained by Notified Bodies during past reviews, and increased requirements relating to medical devices by Competent Authorities. The three-year product review cycle is intended to ensure the following: The device remains equivalent to the devices(s) initially approved. This includes device labelling and other user information supplied with the device. Any significant changes to the product, processes or labelling have been notified to NSAI and approved by NSAI. The company has implemented and kept up-to-date an effective Risk Management Programme. New or revised Harmonised Standards applicable to the device have been considered by the company. Customer complaints have been adequately monitored and addressed by the company, and where appropriate, Reportable Incidents are notified to the Competent Authorities on an on-going basis. As part of the 3-year review, performance/complaints history for the specific device(s) over the 3-year period shall be provided and copies of reportable Incidents and associated Corrective Actions shall be provided. CLINICAL- Detailed performance/complaints history of original devices (plus subsequent amendments) since the last review. Relevant supplementary clinical data specific for the device is required. For IVDs, provide data on up-to-date stability and QC trends to continue to substantiate the continued performance of the product Indicate if the product has been marketed since the initial approval/ last three year review. Note: all the above data requirements must be supplied to NSAI to complete a three year review, irrespective of whether the product has been commercialised or not. By supplying the data requested above, you can help NSAI ensure completion of your product review, and re-issue of your certificates with minimal delay. Europe E-mail: medical.devices@nsai.ie USA E-mail: medical.devices@nsaiinc.com Medical Devices NSAI 1 Swift Square, Northwood, Santry, Dublin 9, Ireland Medical Devices NSAI Inc 402 Amherst St, Suite 100 Nashua NH 03063 USA Phone: +353 (0) 1 807 3929 Toll free (866) 744 6724 Phone (603) 882 4412 GAD-25-22 Rev 5.0 4