Lab 3: Molecular Structures
advertisement

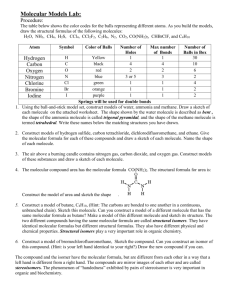
Lab 3: Molecular Structures Introduction There are over a million known organic compounds. The chemical and physical properties depend on the elements present and their order of arrangement. The representation of the bonding in molecules can be represented by the Lewis dot structure. The molecular formula of a molecule may represent the composition of more than one molecule. For example, the molecular formula, C2H6O2, represents two different compounds: CH3CH2OH (ethanol) and CH3OCH3 (dimethyl ether). These two compounds possess different chemical and physical properties. They are isomers (have the same chemical formula but different properties). The formulas for ethanol and dimethyl ether are referred to as condensed structural formulas because information is given on the connectivity of the atoms. Structural isomers are compounds with the same atoms but one or more of the bonds are different. Stereoisomers are compounds with the same atoms and same bonds but the spatial arrangements of the atoms are different. The purpose of this lab is to use molecular models to clarify the theoretical concepts of covalent bonding and molecular structure. Molecular models are designed to reproduce molecular structures in three dimensions. If models are correctly assembled, many subtle feature concerning shapes of molecules (such as dipole moment, polarity, bond angle and symmetry) will become clearer. Each student will be supplied with a molecular model set. Familiarize yourself with the components of the molecular model set with the help of the following tables: Type Short Gray (stiff) Long Gray (flexible) Colour Atom White Black Hydrogen Carbon Red Blue Green Orange Purple Oxygen Nitrogen Chlorine/Flourine Bromine Iodine Table 1: Bonds Use Single Bonds Double and Triple Bonds Table 2: Number of Holes 1 4 5 2 4 1 1 1 Length (mm) 30 45 Atoms Electron-pair Geometry Tetrahedral Trigonal bipyramidal Angular or bent Tetrahedral Maximum # Bonds in Neutral Molecule 1 4 2 3 1 The colour-coded atoms contain from one to five holes drilled at the appropriate bond angles for angular, tetrahedral and trigonal bipyramidal geometries. By leaving some holes vacant, linear, trigonal pyramidal, trigonal planar and other molecular geometries can be obtained as well. The short gray lines are used as single bonds in ball-and –stick models in which the atoms are well Document1 3-1 separated from one another. This makes it easier to see the relative positions of the atoms and to relate a model of a compound to its structural formula. The long gray links are used to construct double and triple bonds or highly strained single bonds. The sizes of the major atoms (H, C, O, and N) correspond closely to actual atomic dimensions. The models can only be used to illustrate bonding as lone pairs are left out. Using the models, proceed through the following exercises, answering the question on the report sheets. The report sheet questions must be answered independently. It is up to the student to make sure they understand the various concepts during the lab. Do not mix up the kit parts with a different set. Part 1 – Covalent Bonding and Molecular Structures You will construct models of a variety of inorganic and organic molecules and then answer questions on the report sheet. For each central atom, choose a ball in which the number of holes equals the total number bonding and non-bonding electron pairs around that atom. Use valence shell electron-pair repulsion (VSEPR) rules to predict the molecular geometries. Sketches should be drawn using dash-line-wedge notation to impart a 3-dimensional representation. By this notation, a solid line represents a bond in the plane of the paper, a wedge represents a bond coming out of or in front of the plane of paper, and a dashed line represents a bond going into or behind the plane of paper. For example, methane, CH4, appears as: H C H H H Complete the table on the report sheet for each molecule or ion. You will be required to: Draw the correct Lewis structure with electrons and any resonance forms. Construct a molecular model for the compounds. Resonance structures require only one model. Sketch the model, using dashed-line-wedge notation. Assign organic functional groups from table 1(Section 1B) Indicate the following for each Lewis structure: o Hybridization on central atom(s) o Electron pair geometry and overall molecular geometry Remember, the placement of the electron pairs determines the structure, but the name of the molecular geometry is based on the positions of the atoms o Polarity Polar means that the molecule has a net dipole Non-polar means that there is no net dipole, either there are no dipoles present or that they cancel our resulting in no net dipole Document1 3-2 Table 3: Some Important Organic Functional Groups Substance Functional Group Substance R Amide (RCONH2) Alcohol (ROH) OH Functional Group O R C NH2 O Aldehyde (RCOH) R Amine (RNH2) C R NH2 H O R Alkane Carboxylic Acid (RCOOH) H R R Alkyl Halide OH O Ester (RCOOR’) C R''' Alkyne C R' C Alkene R R C R'' C C R X R Ether (ROR’) R' O O R' R' O Ketone (RCOR’) R C R' R = rest of molecule Part 2 – Bond Rotations and Conformations On paper a molecule may appear to be static but even solids undergo atomic vibrations due to kinetic energy. Polymers can undergo bond rotation which has an effect on their packing structure and thermal properties (i.e. glass transition temperature and melting point). When bonds on a molecule rotate, this is known as a change in the molecular conformation. Some conformations are higher energy than others. For example, polyethylene chains tend to adopt a zig-zag conformation for the carbon-carbon backbone because this is the lowest energy arrangement. H H H HH H H H C C C C C C H H H C C H H HH H A segment of a polyethylene chain Document1 3-3 Procedure 1. Construct a model of ethane (CH3CH3). Look down the carbon-carbon bond and rotate the carbon-carbon bond until the hydrogens are lined up behind each other. This in known as the eclipsed conformation. Rotate the bond until the hydrogens form a 60° angle (known as the dihedral angle). This is the staggered conformation. 2. Construct a model of cyclopropane (C3H6). Notice how the links must be bent to form the three-membered ring. Such rings are said to be strained. 3. Now construct a model of cyclohexane. Rotate and rearrange the bonds to produce the boat conformation and identify the “flagpole” hydrogen atoms on the report. Convert the boat to a chair conformation and observe the axial and equatorial hydrogen atoms. Part 3 – Structural and Geometric Isomers By using models, one can easily construct a number of molecules for one molecular formula. By comparing the models with each other, identical molecules can be eliminated until the correct number of molecular structures can be determined. For example, the molecular formula C3H6 represents only two molecules that can be constructed. H C H C H H H H Propylene H C C H H C H H C H Cyclopropane Procedure 1. Construct a model of propane (C3H8). Examine your model of propane and note how many indistinguishable hydrogen atoms (hydrogen atoms that cannot be placed in the same location via simple rotation of the molecule) are present in propane. Construct a model of chloropropane (C3H7Cl). How many isomers of chloropropane exist? Sketch them on the report sheet. 2. If a hydrogen atom is replaced with chlorine on chloropropane, how many isomers of dichloropropane (C3H6Cl2) exist? Sketch them on the report sheet. 3. Determine for yourself that there are five isomers with the formula C3H5Cl3. Sketch them on the report sheet. 4. Construct a model of ethene (ethylene, C2H4). Note the rigidity of the molecule and that there is not rotation about the carbon-carbon double bond as there is in ethane or cyclohexane. Construct models to determine how many isomers there are that correspond to formulas (a) C2H3Cl and (b) C2H2Cl2. Draw the different isomers on the report sheet. Document1 3-4 Part 4 – Optical Isomerism Objects that are not superimposable on its mirror image are called chiral, such as the left and right hands. An achiral object is superimposable on its mirror image. Optical active compounds are molecules that have non-superimposable mirror images (i.e. they are chiral molecules). Optical isomers are steroisomers that rotate the polarized light. One isomer will rotate the polarized light clockwise and the other will rotate it counter clockwise. In this part the experiment, the more subtle differences in another type of stereoisomer, the optical isomer, will be studied. Procedure 1. Build two identical models of CH2ClBr. Looking at only one of the models for now, note the plane of symmetry that is defined by the C, Cl and Br atoms. This molecule has an internal plane of symmetry and will be superimposable on its mirror image. Check this fact by lining up the two models so that the atoms are reflected through an imaginary mirror that runs between them. The two molecules are mirror images. Try putting one molecule on top of the other such that all the atoms line up. Are the two molecules superimposable? Is CH2ClBr chiral or achiral? Does interchanging any two atoms (Cl, Br or H) create a new molecule? 2. Using a purple ball to represent fluorine, construct two models of CHClBrF, one that is a mirror image of the other. Check for an interior plane of symmetry. Are these two molecules superimposable? CHClBrF is chiral. 3. Construct models of the compounds given on the report sheet and answer the questions. Document1 3-5 Lab 3: Molecular Structures Name:__________________________ Date_____________ Reminder: the models do not show lone pair electrons, only molecular shape. Show the lone pair electrons in the Lewis structure. Part 1 – Covalent Bonding and Molecular Structure Molecule or Ion 3D Sketch of Model (dash-line-wedge) Atom Hybridization C ________ CHCl3 C ________ CH2O Document1 Lewis Struture (include resonance structures if present) 3-6 Electron Pair Geometry Bond Angle of Central Atom Molecular Geometry Polarity Molecule or Ion Lewis Struture (include resonance structures if present) 3D Sketch of Model (dash-line-wedge) Atom Hybridization NO2¯ N ________ HCCH C ________ Document1 3-7 Electron Pair Geometry Bond Angle of Central Atom Molecular Geometry Polarity Section 1B – Use Table 1 for a list of functional groups Molecule or Ion Lewis Struture (include resonance structures if present) 3D Sketch of Model (dash-line-wedge) Hybridization of Underlined Atom CH3OH C ________ CH3NH2 C ________ CH3COOH C ________ Document1 3-8 Functional Group (Table 3) Bond Angle of Underlined Atom Molecular Geometry of Underlined Atom Strongest Intermolcular Force Molecule or Ion Lewis Struture (include resonance structures if present) 3D Sketch of Model (dash-line-wedge) Hybridization of Underlined Atom CH3OCH3 O ________ CH3COCH3 C ________ CH3CHCH2 Document1 C ________ 3-9 Functional Group (Table 1) Bond Angle of Underlined Atom Molecular Geometry of Underlined Atom Strongest Intermolcular Force Part 2 – Bond Rotation and Conformers 1. Ethane: Circle the conformation of lower energy. Name both conformations. ________________ 2. _____________________ Cyclopropane: I) What is the hybridization of C in cyclopropane? ______________ II) What is the ideal bond angle for this hybridization? ______________ III) Based on the bond angle, do you think cyclopropane is stable relative to larger hydrocarbon rings. 3 Cyclohexane: Draw the “flagpole” hydrogens on the boat conformation of cyclohexane: Document1 3-10 Part 3 – Structural and Geometric Isomers How many groups of indistinguishable hydrogen atoms are present in propane, C3H8? ________________ Sketch of Isomer(s) C3H7Cl C3H6Cl2 C3H5Cl3 Document1 3-11 How many groups of indistinguishable hydrogen atoms are present in ethylene, C2H4? ________________ How many groups of indistinguishable hydrogen atoms are present in chloroethylene, C2H3Cl? ________________ Sketch of Isomer(s) C2H3Cl C2H2Cl2 (Hint 3 isomers) Document1 3-12 Part 4 – Optical Isomerism Circle the correct answer 1. Bromo-chloro-methane: Are the two molecules of CH2ClBr superimposable on each other? Yes No Is CH2ClBr chiral or achiral? Chiral Achiral Does interchanging an H atom with either the Cl or Br make a molecule identical to the original molecule? Yes No 2. Bromo-chloro-fluoro-methane Are the two molecules of CHClBrF superimposable on each other? Yes No Is CHClBrF chiral or achiral? Chiral Achiral Does interchanging an H atom with either the Cl or Br make a molecule identical to the original molecule? Document1 3-13 Yes No Gylcine H2NCH2COOH Alanine CH3NH2CHCOOH Document1 3-14 (complete) Optical Active? (yes/no) Sketch of Mirror Image Number of Chiral Centres Sketch (Given) Internal plane of symmetry? (yes/no) Compound Is Mirror Image Superimposable? (yes/no) 3. Construct the following molecules and complete the table: Conclusions: Document1 3-15