grl52235-sup-0001-README
advertisement

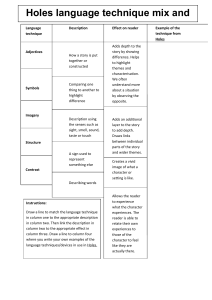
Auxiliary Material for Contribution of Multidomain Titanomagnetite to the Intensity and Stability of Mars Crustal Magnetic Anomalies Stefanie Brachfeld1, David Cuomo1, Lisa Tatsumi-Petrochilos2, Julie Bowles3, Deepa Shah1, Julia Hammer2 (1. Department of Earth and Environmental Studies, Montclair State University, Montclair, NJ, 2. Department of Geology and Geophysics, University of Hawai’i at Manoa, Honolulu, Hawai’i, 3. Department of Geosciences, University of Wisconsin-Milwaukee, Milwaukee, WI) Geophysical Research Letters Introduction Table 1 (TS01.xlsx) contains the bulk compositions of the M-type and T-type basalts described in the main text (upper half of table) and the CIPW normative mineralogy (lower half of table), and the compositions of the materials on which they were patterned. M-type composition is patterned on Chassigny A [Johnson et al., 1991]. T-type composition is identical to M-type with the exception of Fe (poorer), Al (richer), and Ca (slightly richer) [Bowles et al., 2009]. The Ttype composition is similar to Medicine Lake Highland basalt 82-66 [Sisson and Grove, 1993], a high alumina basalt that is consistent with thermal emission spectrometer constraints on the composition of Mars' surface [Hamilton et al., 2001]. Compositions reported for MAm-34 and MB2-50 are EMPA analyses of fused beads of samples created in the high-temperature step for M-type and T-type starting materials in [Petrochilos, 2010]. Compositions reported for A*mod and B2 are based on masses of oxide reagents used to prepare the samples. The program MAGMA [Wohletz 1999] was used to calculate the CIPW norms. FeO and Fe2O3 were computed from FeO(t) using the method of Kilinc et al. [1983] assuming 1200 ˚C and fO2 at the QFM buffer for the samples with superscripts b and c [Bowles et al., 2009], and 1070 ˚C and QFM for the rest. The norm was calculated using the LEPR applet by OFM research (ofmresearch.org). Table 2 (TS02.xlsx) contains rock magnetic parameters for the M-type and T-type samples described in the main text. These include magnetic susceptibility (χ), natural remanent magnetization (NRM), anhysteretic remanent magnetization (ARM), the ratio NRM/ARM, saturation magnetization (MS), saturation remanence (MR), the ratio MR/MS, coercivity (HC), coercivity of remanence (HCR), the ratio HCR/HC, Curie temperature (TC), and low temperature order-disorder transition temperature (TL). NRM and ARM were measured on a 2G-Enterprises Model 755 magnetometer at Lehigh University and on an AGICO JR6 Spinner magnetometer at Montclair State University. ARM was imparted in a 100 mT peak alternating field and a 98 µT DC bias field. Stepwise alternating field (AF) demagnetization data were used to calculate the median destructive field (MDF) of the ARM. Magnetic hysteresis measurements were made on a Princeton Measurements Corp. micro-Vibrating Sample Magnetometer (VSM) model 3900-04 at Montclair State University, NJ. Hysteresis loops were measured in a peak field of 1 T and field increments of 5 mT and corrected for the paramagnetic contribution to the induced magnetization. The hysteresis parameters MS, MR and HC were determined from the paramagnetic-corrected data. HCR was determined through the DC-demagnetization of a saturation isothermal remanent magnetization imparted in a 1 T field. Curie temperatures (TC) were measured on the VSM in a flowing helium gas atmosphere. We monitored the induced magnetization in a 50 mT applied field as a function of temperature from 25-700 ˚C. Low temperature order-disorder transitions were analyzed on a Quantum Design Magnetic Properties Measurement System (MPMS) at the Institute for Rock Magnetism (IRM), University of Minnesota. An isothermal remanent magnetization (MR) was imparted at 20 K in a 2.5 T field and the intensity of remanence monitored during warming to 300K. Table 3 (TS03.xlsx) contains electron microprobe analysis of individual oxide crystals in M-type and T-type samples described in the main text. All electron microprobe analyses were conducted on a JEOL JXA-8500F Field Emission Hyperprobe at the University of Hawai’i at Mānoa. Instrument calibration settings and standards used are reported in Appendix G of Petrochilos [2010]. FeO(t) was differentiated into Fe2O3 and FeO using the method of Droop [1987], and these were used to recalculate the corrected oxide total. The number of oxygen atoms per formula unit is 3 for ilmenite and 4 for spinels. Calculations include cations per formula unit and mol% of end-member mineral compositions. Figure 1 (FS01.tif) shows low temperature thermomagnetic curves for MAm-9E, MAm-11E, MAm-16E, MB2-9E, MB2-12E, and MB2-16E. Samples were analyzed on a Quantum Design Magnetic Properties Measurement System (MPMS) at the Institute for Rock Magnetism (IRM), University of Minnesota. An isothermal remanent magnetization (MR) was imparted at 20 K in a 2.5 T field. Field-cooled (FC) samples were cooled from 300 K to 20 K in the presence of the 2.5 T field. Zero-field-cooled (ZFC) samples were cooled from 300 K to 20 K in zero applied field. For both FC and ZFC measurements the intensity of remanence was monitored during warming from 20 K to 300 K. Low temperature order-disorder transitions (TL) are reported in Table TS02. 1. TS01.xlsx. Bulk Compositions (wt% oxide) of Starting Materials and CIPW Normative Mineralogy 1.1 Column “Oxide” (upper half) and “Normative Mineralogy” (lower half) 1.2 Column “Chassigny A”, wt% oxide and normative mineralogy for Mars basaltic meteorite Chassigny A 1.3 Column “A*”, wt% oxide and normative mineralogy for composition A* 1.4 Column “A*mod”, wt% oxide and normative mineralogy for composition A*mod 1.5 Column “MAm-34”, wt% oxide and normative mineralogy for sample MAm-34 1.6 Column “Terrestrial,” wt% oxide and normative mineralogy for terrestrial arc basalt 1.7 Column “B2”, wt% oxide and normative mineralogy for composition B2 1.8 Column “MB2-50”, wt% oxide and normative mineralogy for sample MB2-50 2. TS02.xls Rock Magnetic Properties of Synthetic Basalts 2.1 Column “Sample ID” 2.2 “Anneal days”, days, days held at 650 ˚C 2.3 Column “χ”, m3/kg, low field magnetic susceptibility 2.4 Column “NRM”, mAm2/kg, natural remanent magnetization interpreted as a TRM acquired in the 35 µT laboratory field 2.5 Column “ARM”, mAm2/kg, anhysteretic remanent magnetization induced in a 98 µT DC bias field and 100 mT peak alternating field 2.6 Column “NRM/ARM,” NRM normalized by ARM 2.7 Column “MS”, Am2/kg, saturation magnetization 2.8 Column “MR”, Am2/kg, saturation remanence 2.9 Column “MR/MS”, saturation remanence normalized by saturation magnetization 2.10 Column “HC”, mT, coercivity 2.11 Column “HCR”, mT, coercivity of remanence 2.12 Column “HCR/HC,” coercivity of remanence normalized by coercivity 2.13 Column “TC”, degrees Celsius, Curie temperature 2.14 Column “TL”, degrees Kelvin, low temperature order-disorder transition temperature 3. TS03.xlsx. Composition of oxide phases in wt % oxide, cations per formula unit, and fraction of end-members 3.1 Column “Sample/grain/spot,” Sample identification, grain identification, and analytical spot identification 3.2 Column “Anneal time,” days, days held at 650 ˚C 3.3 Column “SiO2”, wt % 3.4 Column “TiO2”, wt % 3.5 Column “Al2O3”, wt % 3.6 Column “Cr2O3”, wt% 3.7 Column “FeO(t)”, wt%, total iron 3.8 Column “MnO”, wt% 3.9 Column “MgO”, wt% 3.10 Column “Total”, wt%, sum of wt% oxides 3.11 Column “Fe2O3”, wt%, FeO(t) differentiated into Fe2O3 following Droop [1987] 3.12 Column “FeO”, wt%, FeO(t) differentiated into FeO following Droop [1987] 3.13 Column “Corr. Total”, sum of oxides recalculated using Fe2O3 and FeO 3.14 Column “O”, number of oxygen atoms per formula unit (3 for ilmenite, 4 for spinel) 3.15 Column “Si”, number of Si cations per formula unit 3.16 Column “Ti”, number of Ti cations per formula unit 3.17 Column “Al”, number of Al cations per formula unit 3.18 Column “Cr”, number of Cr cations per formula unit 3.19 Column “Fe”, number of Fe cations per formula unit (reported as total Fe) 3.20 Column “Mn”, number of Mn cations per formula unit 3.21 Column “Mg”, number of Mg cations per formula unit 3.22 Column “Fe3+”, number of Fe3+ cations per formula following Droop [1987] 3.23 Column “Fe2+”, number of Fe2+ cations per formula following Droop [1987] 3.24 Column “Fe2TiO4 /FeTiO3”, mol%, mol % of ülvospinel or ilmenite end-member 3.25 Column “FeAl2O4”, mol%, mol % of hercynite end-member 3.26 Column “MgFe2O4 /MgTiO3,” mol%, mol % of jacobiste or geikielite end member 3.27 Column “FeCr2O4”, mol % of chromite end-member 3.28 Column “Fe3O4 / Fe2O3”, mol % of magnetite or hematite end-member 3.29 Column “Phase”, mineral name 4. FS01.tif. Field cooled (FC) and zero-field-cooled (ZFC) saturation remanence vs. temperature (MR-T) curves measured from 20 to 300 K for MAm-9E, MAm-11E, MAm-16E, MB2-9E, MB2-12E, and MB2-16E. References Bowles, J., J. Hammer, and S. Brachfeld (2009), Magnetic and petrologic characterization of synthetic Martian basalts and implications for the surface magnetization of Mars, J. Geophys. Res., E10003, doi:10.1029/2009JE003378. Droop, G. T. R. (1987), A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria, Mineral. Mag., 51, 431-435, doi:10.1180/minmag.1987.051.361.10. Hamilton, V. E., M. B. Wyatt, H. Y. McSween Jr., and P. R. Christensen (2001), Analysis of terrestrial and Martian volcanic compositions using thermal emission spectroscopy: 2. Application to Martian surface spectra from the Mars Global Surveyor Thermal Emission Spectrometer, J. Geophys. Res., 106, 14,733–14,746, doi:10.1029/2000JE001353. Johnson, M.C., M.J. Rutherford, and P.C. Hess (1991), Chassigny petrogenesis melt compositions, intensive parameters, and water contents of Martian (?) magmas, Geochim. Cosmochim. Acta 55, 349–366. Kilinc, A., I.S.E. Carmichael, M.L Rivers, and R.O. Sack (1983), The ferric-ferrous ration of natural silicate liquids equilibrated in air. Contrib. Mineral. Petr., 83, 136-140, doi:10.1007/BF00373086. Petrochilos, L.T. (2010), Experimental and analytical studies of titanomagnetite in synthetic and natural samples, Masters thesis, University of Hawaii Manoa, 157 pp. Sisson, T.W. and T.L. Grove, T.L. (1993) Experimental investigations of the role of H2O in calcalkaline differentiation and subduction zone magmatism. Contrib. Mineral. Petr., 113, 143-166. Wohletz, K. H. (1999), MAGMA: Calculates IUGS Volcanic Rock Classification, Densities, and Viscosities. Los Alamos National Laboratory computer code LA-CC 99-28, Los Alamos New Mexico.