Lesson 1: Gases: Properties and Behaviour
advertisement
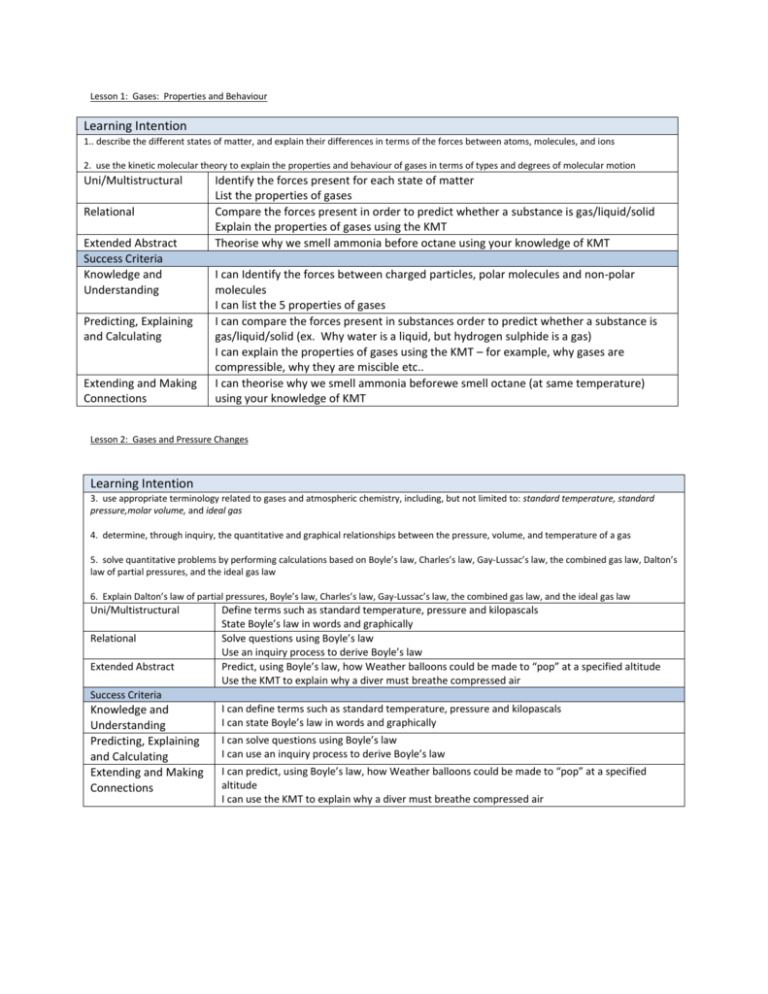
Lesson 1: Gases: Properties and Behaviour Learning Intention 1.. describe the different states of matter, and explain their differences in terms of the forces between atoms, molecules, and ions 2. use the kinetic molecular theory to explain the properties and behaviour of gases in terms of types and degrees of molecular motion Uni/Multistructural Relational Extended Abstract Success Criteria Knowledge and Understanding Predicting, Explaining and Calculating Extending and Making Connections Identify the forces present for each state of matter List the properties of gases Compare the forces present in order to predict whether a substance is gas/liquid/solid Explain the properties of gases using the KMT Theorise why we smell ammonia before octane using your knowledge of KMT I can Identify the forces between charged particles, polar molecules and non-polar molecules I can list the 5 properties of gases I can compare the forces present in substances order to predict whether a substance is gas/liquid/solid (ex. Why water is a liquid, but hydrogen sulphide is a gas) I can explain the properties of gases using the KMT – for example, why gases are compressible, why they are miscible etc.. I can theorise why we smell ammonia beforewe smell octane (at same temperature) using your knowledge of KMT Lesson 2: Gases and Pressure Changes Learning Intention 3. use appropriate terminology related to gases and atmospheric chemistry, including, but not limited to: standard temperature, standard pressure,molar volume, and ideal gas 4. determine, through inquiry, the quantitative and graphical relationships between the pressure, volume, and temperature of a gas 5. solve quantitative problems by performing calculations based on Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, Dalton’s law of partial pressures, and the ideal gas law 6. Explain Dalton’s law of partial pressures, Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, and the ideal gas law Uni/Multistructural Relational Extended Abstract Define terms such as standard temperature, pressure and kilopascals State Boyle’s law in words and graphically Solve questions using Boyle’s law Use an inquiry process to derive Boyle’s law Predict, using Boyle’s law, how Weather balloons could be made to “pop” at a specified altitude Use the KMT to explain why a diver must breathe compressed air Success Criteria Knowledge and Understanding Predicting, Explaining and Calculating Extending and Making Connections I can define terms such as standard temperature, pressure and kilopascals I can state Boyle’s law in words and graphically I can solve questions using Boyle’s law I can use an inquiry process to derive Boyle’s law I can predict, using Boyle’s law, how Weather balloons could be made to “pop” at a specified altitude I can use the KMT to explain why a diver must breathe compressed air Lesson 3: Gases and Temperature Changes Learning Intention 4. determine, through inquiry, the quantitative and graphical relationships between the pressure, volume, and temperature of a gas 5. solve quantitative problems by performing calculations based on Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, Dalton’s law of partial pressures, and the ideal gas law 6. Explain Dalton’s law of partial pressures, Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, and the ideal gas law Uni/Multistructural Relational Extended Abstract Success Criteria Knowledge and Understanding Predicting, Explaining and Calculating Extending and Making Connections State Charles’s and Gay-Lussac’s law mathematically, in words and graphically Define absolute zero Convert between Celcius and Kelvin Solve questions using the Laws Predict the method to perform an inquiry on either law Use the KMT to explain these gas laws Hypothesize how your knowledge of Gay-Lussac’s law could influence how to safely store compressed gas cylinders Squash balls need to be ‘warmed up’ by rallying before beginning a game, comment on the gas laws involved I can State Charles’s and Gay-Lussac’s law mathematically, in words and graphically I can Define absolute zero I can Convert between Celcius and Kelvin I can solve mathematical questions using the Laws I can develop an experimental method (demo) to demonstrate the law I can Use the KMT to explain these gas laws I can Hypothesize how your knowledge of Gay-Lussac’s law could influence how to safely store compressed gas cylinders I can explain, using gas laws, why Squash balls need to be ‘warmed up’ by rallying before beginning a game, (page 537 #14 too) Lesson 4 – The Combined Gas Law Learning Intention 5. solve quantitative problems by performing calculations based on Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, Dalton’s law of partial pressures, and the ideal gas law 6. Explain Dalton’s law of partial pressures, Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, and the ideal gas law 7. Explain Avogadro’s hypothesis and how his contribution to the gas laws has increased our understanding of the chemical reactions of gases Uni/Multistructural Relational Extended Abstract Success Criteria Knowledge and Understanding Predicting, Explaining and Calculating Extending and Making Connections State the Combined and Avogadro’s law mathematically and in words Define STP and SATP Solve molar volume questions using Avogadro’s law Solve questions using the combined gas law Use the KMT to explain the laws Predict how to calculate the molar mass of an unknown gas using Avogadro’s law and molar volume Create an analogy to explain the Laws to a small child who is young I can state the Combined and Avogadro’s law mathematically and in words I can define STP and SATP I can solve molar volume questions using Avogadro’s law I can solve questions using the combined gas law I can use the KMT to explain the laws I can predict how to calculate the molar mass of an unknown gas using Avogadro’s law and molar volume I can create an analogy to explain the Laws to a small child who is young Lesson 5 – 2 parts Ideal gas law Learning Intention 5. solve quantitative problems by performing calculations based on Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, Dalton’s law of partial pressures, and the ideal gas law 6. Explain Dalton’s law of partial pressures, Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, and the ideal gas law Uni/Multistructural Relational State the Ideal gas law Using the ideal gas law, solve basic problems, solve for density of a gas, solve for molar mass of a gas, and solve combined problems (such as molecular formula) Extended Abstract Success Criteria Knowledge and Understanding Predicting, Explaining and Calculating Extending and Making Connections I can state the Ideal gas law I can use the ideal gas law to solve basic problems, solve for density of a gas, solve for molar mass of a gas, and solve combined problems (such as molecular formula) Learning Intention 5. solve quantitative problems by performing calculations based on Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, Dalton’s law of partial pressures, and the ideal gas law 6. Explain Dalton’s law of partial pressures, Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, and the ideal gas law Uni/Multistructural Relational Extended Abstract Success Criteria Knowledge and Understanding Predicting, Explaining and Calculating Extending and Making Connections State Dalton’s Law of partial pressure State conditions where a gas may act not ideally Solve gravimetric stoichiometry problems using the gas laws Choose the appropriate gas law to solve the particular problem I can state Dalton’s Law of partial pressure I can state conditions where a gas may act not ideally I can solve gravimetric stoichiometry problems using the gas laws I can choose the most efficacious gas law for solving a problem