SWP06.10_BMIF_Zeiss PALM Elyra Microscope
advertisement

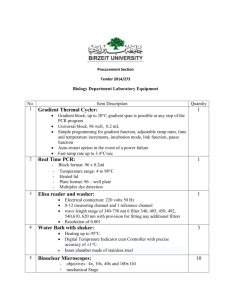
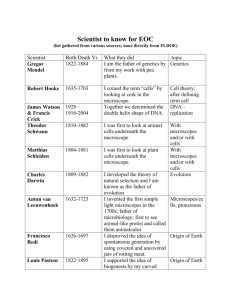
OHS026 Safe Work Procedure Faculty/Division Biomedical Imaging Facility (BMIF) School/ Divisional Unit Analytical Centre: DVC Research Document number Initial Issue date Current version SWP06.10 10/05/2010 1 Current Version Issue date 10/05/2010 Next review date May 2012 The Writing Safe Work Procedures Guideline (OHS027) should be consulted to assist in the completion of this form. Safe Work Procedure Title and basic description Title: Zeiss Elyra (PAL-M) microscope safe work procedure Description: Safe work procedure for Zeiss Elyra (PAL-M) microscope Associated risk assessment title and location: Confocal Microscopy Risk assessment ___________________________________________________________________________________________________________ ___________ Page 1 of 5 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007 Describe the activity or process ___________________________________________________________________________________________________________ ___________ Page 2 of 5 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007 Before work commences: Users must have received authorised trained and the training documented (Training is available through Henry Haeberle ext. 51726 and Astrid Magenau ext. 51304). You must have read and understood this SWP, the associated Risk Assessment (Document #) and the user notes (operation guide) for this equipment. Samples brought into the imaging facility must be clean, dry, safe and enclosed (e.g. mounted slide). Live cells must be transported correctly. Where applicable, OGTR regulations must be complied with and the specimens suitably contained. The microscopy facility is a PC1 only facility, no PC2 level specimens may be transported into the facility. The contact person must be consulted before bringing any chemicals into this facility. (Copies of all relevant documents are located by each microscope in Room LG 22 and LG24) USE OF METAL HALOGENIDE LAMP (X-CITE 120Q) Before turning on, check that the accumulated time of use is less than 2500 hours (according to the X-CITE). If the accumulated time exceeds 2500 hours do not use equipment but notify BMIF Microscopy staff, who will replace the lamp. Before turning on, check that the lamp has been off for at least 20 minutes (according to the BMIF booking system). If it has been turned off within the last 20 minutes you must wait until it has been off for 20 minutes before reigniting the lamp. Before turning the X-Cite off, check the booking calendar to see if someone is using it after you. If someone is using it within up to 1 hour of you finishing, leave it on. Otherwise, turn it off so long as it has been on for at least 20 minutes (see below). Do not turn the Mercury Lamp off within 20 minutes of being switched on. If you finish your work within 20 minutes (and noone is booked to use it in the next hour), you must wait until it has been on for 20 minutes before turning it off. USE OF THE MICROSCOPE Note: This document does not constitute a manual or training guide. Users of this equipment must receive authorised training and use this Safe Operating procedure in conjunction with the user notes for this equipment and the risk assessment for this equipment. Follow the respective TIRF microscope user notes for operation of the computers, microscopes, scanner and lasers. Keep the reflected light shutter closed when the X-Cite light source is not required. Do not activate laser light through the objectives unnecessarily. Never look directly into the light (laser light or light from the X-Cite) coming through the objective lens. Do not attempt to view through eyepieces whilst laser light is passing through objectives (a shutter normally physically prevents the laser light passing through the eyepieces). With the reflected light shutter and the laser shutter closed and the lasers not activated, select and add a small drop of the correct immersion oil. Wash your hands after using immersion oil. With the shutters closed, the lasers not activated and the microscope stage lowered (upright microscopes) or the objective turret lowered (inverted microscopes), put your sample on the microscope stage. With either Brightfield or fluorescence light (reflected light shutter open), adjust the stage/objective height to bring your specimen into focus, by viewing through the eyepieces. Take particular care not push the objective lens onto the slide beyond the focal point. Ensure the shutters are closed and lower the stage (upright microscopes) or the objective turret (inverted microscopes) before adding or removing a specimen on the microscope stage. Assure that there are no spills of immersion media or cell culture media. Clean the objective lens and the stage after use. This is also therefore how the microscope should be left when you have finished. Acquire images as desired using lasers and computer. ADDITIONAL SOP REQUIREMENTS FOR USE Maintain correct posture whilst working at the microscope and computer (refer to http://www.hr.unsw.edu.au/ohswc/workerscomp/wc_workstation.html ). Do not work at the microscope / computer for more than 2 hours without taking a break. Fixed specimens must be mounted on a microscopy slide and be sealed, clean and dry. This means that slides must be free from excess mounting media and that nail varnish must have had at least 60 minutes to set. Live cells must be transported correctly. Where applicable, OGTR regulations must be complied with and the specimens suitably contained. Chemicals are not to be brought into the fluorescence microscopy facility without prior consent from facility management. Microscope slides are to be carried in an appropriate container, and broken slides or coverslips are to be disposed of immediately in the sharps containers provided. ___________________________________________________________________________________________________________ ___________ Page 3 of 5 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007 List all resources required including plant, chemicals, personal protective clothing and equipment, etc Lab coats and gloves if working with chemicals or GMOs. List potential hazards and risk controls including specific precautions required Postural damage- Users shall be informed of OH&S posture regulations and shall work a most 2hours. Eye damage from UV light – Users shall not look directly into objectives, and minimize light emission from objectives, and keep plastic UV shields around objectives when possible. Eye damage from lasers (UV and visible lasers) - Users shall not look directly into objectives, and minimize laser light emission from objectives. Mercury vapour exposure- users will not operate with mercury bulbs beyond rated range, users will evacuate scope in case of mercury lamp explosion and inform emergency personnel. Cuts from broken glass (slides / coverslips)- users shall know location of first aid. List emergency shutdown instructions In the software, click on ‘turn off lasers’ Close the software (click the [X] in the top right corner) o Shut down computer (by clicking Start Shutdown) Turn off the microscope by pressing the button on the left-hand side of the microscope. Turn off the power point labeled with ‘lasers’. Turn off the switch on the front of the X-Cite 120Q. List clean up and waste disposal requirements All GMOs must be transported in appropriate containers. DO NOT USE SOLVENTS ON THE OBJECTIVES. Only use ethanol on objective glass. Clean up any spills on stage with an Ethanol soaked tissue. Do not clean up spills on the microscope. Turn off power and contact BMIF personal. List legislation, standards and codes of practice used in the development of the SWP 2243.3, 2243.1 Supervisory approval, training, and review Supervisor: Chris Marjo Signature: Plant custodian: Signature List competency required – qualifications, certificates, licensing, training - eg course or instruction: ___________________________________________________________________________________________________________ ___________ Page 4 of 5 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007 SWP review date: Responsibility for SWP review: ___________________________________________________________________________________________________________ ___________ Page 5 of 5 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007