OBJECTIVES (for lecture U1L13 and U1L14): 1. What are meant by
advertisement

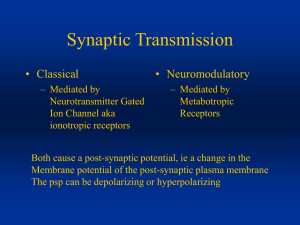
OBJECTIVES (for lecture U1L13 and U1L14): 1. What are meant by the "presynaptic" and "postsynaptic" parts of a chemical synapse? What are their respective roles? 2. Draw a diagram showing a cross section of a neuromuscular synapse (synapse between a somatic motor neuron and a voluntary muscle fibre). What is the function of this synapse? 3. On the diagram above mark the location of voltage-gated Ca2+ channels, synaptic vesicles, nicotinic acetylcholine receptors and voltage-gated sodium channels. What is the function of each of these in the process of synaptic transmission? 4. What is meant by the term ligand-gated ion channel? What are the key features of a ligand-gated ion channel that affect its function? Why are some ligand-gated ion channels excitatory and others inhibitory? Explain. 5. Outline the sequence of events from the arrival of a propagated action potential at the nerve terminal to the muscle cell action potential? 6. What happens to intracellular Ca2+ when the nerve action potential reaches the nerve terminal? What is the mechanism by which this rapid brief rise in intracellular Ca2+ occurs? 7. How does this rapid brief rise in intracellular Ca2+ affect the exocytosis of neurotransmitter? Why? 8. Explain in your own words what is meant by the vesicle hypothesis for quantal synaptic transmission. 9. What effect on synaptic transmission would be expected if the concentration of calcium ions in the extracellular fluid were reduced? 10. What is meant by the term excitatory postsynaptic potential or endplate potential? How do such potentials arise? 11. What is a spontaneous miniature endplate potential? What synaptic event is thought to cause a spontaneous miniature endplate potential? 12. Explain the role of acetylcholinesterase in terminating the endplate potential. 13. Outline the process by which acetylcholine is recycled at the neuromuscular junction. BMED 2801 – Lecture 13: Principles of Synaptic Transmission In this lecture: Propagation action potentials Synaptic transmission Propagation of action potentials can occur in two ways: Continuous propagation Saltatory propagation - - Continuous propagation occurs in unmyelinated axons, it’s just an axon membrane found in pain fibres, in our bodies, in simpler organisms, it’s a common form of synaptic transmission, it’s found more in simpler organisms because its smaller and the distances the signals have to travel is much shorter so the time factor isn’t a such problem. Continuous propagation works by having voltage gated sodium channels and voltage gated potassium channel spread all along the length of the axon. The action potential might start to appear at the axon hillock, but once it gets going the depolarisation spreads by local circuit currents to spread the depolarisation to the next area of membrane. When this happens, the Hodgkin’s cycle gets started a little further downstream, this is a short while later (the process is like a rolling depolarisation, moving along the length of the axon). How come the action potential doesn’t turn around and go back in the same direction? The electric currents, i.e. the local circuit currents are passively spreading the charge in both directions. Why doesn’t the Hodgkin cycle start down later? Property of the voltage gated sodium channels - - - - The sodium channels are inactivated (close state, open state, inactivated state), when the membrane has become depolarised for a while, the channels become inactivated i.e. the “ball and chain structure” plugging the channel so that these channels down “here” that were recently activated, are now inactivated. Whilst this area may be depolarised by the local circuit current, the Hodgkin’s cycle can’t happen here until the membrane repolarises and then a millisecond or two has to go by before it goes from the inactivated state (the closed state) so then the sodium channels can engage once more in Hodgkin’s cycle, when the depolarisation comes along. Meanwhile, the action potential has moved on further along the membrane, the membrane behind it has started to repolarise as a result of the voltage gated potassium channel opening, so this system of voltage gated potassium channels, the way in which they open, allows a kind of rolling depolarisation which is like a wave which moves along the axon from one end to the other, with the area behind becoming inactivated and then repolarised, the area in front becoming depolarised by the spreading charge, and then entering the Hodgkin’s cycle to get the whole thing going again so it rolls down the length of the axon. This limits the rate it can propagate; it is a wave, however it is a wave that moves relatively slowly, approximately a tenth of a metre per second. This seems fast enough, but not as fast as the body needs it to get fast signalling going for moving arms and legs etc. The things that limit the rate of propagation through non-myelinated axons are these things... Here’s the electric current that’s generated, the local circuit current, the amplitude of depolarisation diminishes with distance, V m is membrane potential, and the depolarised Vm represented “here” from resting, diminishes in amplitude as it moves along. - - - - - That’s because of the electrical properties (talked about last time). The electrical properties can be modelled by an electrical circuit, and this electrical circuit consists of a good conductor on the outside, represented by “this wire”, electrical conduction through the extracellular fluid is easy because there’s a lot of fluid there to carry the current. On the inside, there is a resistance to the flow of circuit through the axoplasm, we refer to these symbols for resistors as the axoplasmic resistance because the cytoplasm is very thin, long tube. The greater the resistance the slower the current, the less current that’ll move down along the membrane, and the slower it’ll take to depolarise the next bit of membrane. The axoplasmic resistance is depended upon how thick the diameter of the axon is. The bigger the diameter, smaller resistance, the smaller the diameter, the higher the resistance i.e. slower spread of depolarising current. There is also leakage to the membrane (the leakage channels), sodium potassium leakage channels meaning that the depolarisation starts “here” some of it is going to leak and short circuit before it gets further down the axon. These resistor symbols across the membrane represent the fact that there are leakage channels that allow some of the current to leak out as it goes along. The less resistant those channels are to leakage of current, the less current that’ll move along the axon, the more of it that’ll leak out. The capacitance symbol, all connected together in the circuit, refers to the fact that the membrane is a thin insulator separating two electrical conductors, and so, charge will cling to either side of the membrane in the same way the charge will cling to the conductors on either side of the capacitor. It is a charge storage device, and the more membrane there is, the more stored charge, the longer it takes to depolarise that stored charge, or to discharge the membrane capacitance. When a depolarisation starts here, a bigger current is needed to run for longer in order to raise the membrane potential along here because of all the stored charge of the membrane. How can we get around these electrical problems that slow down propagation of action potential? - - - Above: cross section through squid’s nerve. The squid giant axon is 0.8mm, compare these to the relative surrounding axons, they are tiny. A big diameter has very little axoplasmic resistance. Electric currents move through the axon more easily, so that the depolarisation spreads further down the axon faster to get the action potential to roll along this big diameter faster than it will along the thin diameter axons. The axoplasmic resistance is reduced, the charge spreads faster, the action potential propagates faster. Above right: cross section through mammalian axon – has connective tissue around it to protect the nerves, the little white spots are the axoplasm mammalian axons. Having a large diameter in the axon, like that in the squid is not possible in complex organisms (such as humans), where there are many channels of information that must work in parallel. Making big diameter axons with low axoplasmic resistance is not really an option. The solution is to change the other electrical properties of the axon and that involves myelination. The myelin sheath is an area along the length of the axon where a glial cell called a shwann cell wraps around the axon. The schwann cell is an accompanying cell, during embryonic development, the schwann cell starts to wrap itself around the axon, until there are many layers of myelin sheath, it’s double membrane surrounds the axon numerous times until it produces a multilayered membrane sheath that is the myelin, and that is what makes the nerves white, the “fat” from the myelin. The schwann cell sits there and maintains the myelin sheath. - - - - In a myelinated axon, the voltage gated Na+ channel migrates to the spaces between the myelin, the myelinated internodes. Between them are the nodes, the nodes of Ranvier, where the voltage gated Na+ channels cluster in the membrane of the axon. These are the active parts now, instead of having action potential rolling along the axon, now the voltage gated Na+ channels to amplify the signal and the node of Ranvier (the amplification stations), at spaces of about 1mm apart or so. Okay so... we have a voltage gated Na+ channels that generate an action potential, the Hodgkin cycle occurs here, not many voltage gated channels in between, so the action potential then causes a passive spread of depolarisation. The next node of Ranvier, where the signal is amplified again by another group of voltage gated sodium channels and so on and so on. The effect of this is that the signal gets amplified at each node of ranvier, it spreads very rapidly between those nodes of ranvier and it moves more rapidly along the axon. What does this mean electrically? - The myelinated internode region, the axoplasmic resistance isn’t changed but the leakage channels are largely covered up, (shown by the resistor symbol as long and thin). - So very little charge can leak across the membrane so more of the current can then move along the axon. - The capacitance property of the membrane has been greatly reduced, because there’s this big insulating layer around the membrane and so the charge on the inside is separated by a hundred times more distance than if it was if the axon was bare. - The charge is separated much more, (opposite charges attract, the further away, the less attraction), the attraction diminishes with the square of the distance, so by increasing the separation by a hundred fold, there will be almost no attraction to make those charges cling to the membrane. - The charge concentrates at the nodes of ranvier where they can cling to each other across the membrane. - Charges stored in the space in between the internode region is an area where depolarisation spreads through these local circuit currents move in a much less diminished way to the next node of ranvier where they can rapidly depolarise the next node of ranvier to trigger the Hodgkin’s cycle to be generated by the voltage gated sodium channels that are concentrated there. i.e. In saltatory propagation Voltage-gated Na+ channels are concentrated at the axon hillock and Nodes of Ranvier The Hodgkin Cycle is triggered at one Node after another. This amplifies the signal The signal travels passively as an electrical current between Nodes. The thick myelin insulation of the Internode allows the local circuit current to spread much further and faster than in un-myelinated fibres What happens if the membrane is demyelinated? i.e. if the axon sheath is stripped away - This can happen in various kinds of toxins, can cause damage to the schwann cells, autoimmune anti-bodies, in the CNS can cause damage to the analogous oligodendrocytes. - - i.e. If the myelin sheath is lost, when all of the leakage channels are exposed, as the depolarisation starts to spread down the axon, much of it’ll leak out like in an unmyelinated axon shown previously. Losing current, leaking out, “short circuiting”, there’s also a lot more stored charge in the membrane, the charge can cling across the membrane, so there’s a lot of stored charge that needs to be discharged before the depolarising current can reach the next group of voltage gated sodium channels. Losing some internodes, some of the myelin sheath of a part of the axon can lead to firstly, a slowing down of action potential propagation (if there’s damage to the myelin sheath, there’ll always be slowing of propagation). If this gets bad enough the depolarising current may not reach a sufficient depolarisation to trigger the Hodgkin cycle further down and it can get action potential block. When that happens, signals don’t pass through the blocked area and you can either get loss of sensation in the peripheral or loss of control of the muscles. Increases permeability of membrane to ions in the demyelinated internode regions Increased membrane capacitance in the denervated internode region Reduced membrane electrical resistance leads to loss of amplitude of signal Inevitable slowing of action potential propagation through regions with high membrane capacitance Synaptic Transmission: - When the action potential propagates down to a synapse, the voltage gated calcium channels open in the nerve terminal (end of the nerve) and allows calcium into the cell. - Calcium diffuses in, (because calcium in the blood is about 1~2mM, inside the cell, it is a thousand fold lower in concentration), it is a strong chemical driving force to drive calcium into the nerve terminal when those voltage gated calcium channels open. - Calcium rushes in and raises the intracellular calcium, the rise in the intracellular triggers the release of neurotransmitter, the neurotransmitter is a chemical, and is often ligand in this case (ligand gated ion channels are the receptors that they bind to). - The neurotransmitter then diffuses across the short gap between the nerve terminal and the post synaptic cell where it binds to and activates a receptor protein on the membrane of the postsynaptic cell. - In the case of the neuromuscular junction (NMJ) it depolarises the postsynaptic cell. - Finally, the neurotransmitter gets removed from the synaptic cleft so that another signal can pass along and communicate this information by converting depolarisation of the action potential into transmitter chemical release, into activation receptor channels, into further depolarisation of the target cell. i.e. Nerve AP depolarises nerve terminals Voltage-gated Ca2+ channels in terminal open leading to increase in [Ca 2+]i Increase in [Ca2+]i triggers release of transmitter into the synaptic cleft Transmitter activates postsynaptic ligand-gated ion channels, opening them Altered membrane current depolarises or hyperpolarises postsynaptic cell Neurotransmitter is removed from synaptic cleft - - - So above, we have the end of an axon i.e. the end of the nerve terminal of the motor neuron. There are synaptic vesicles (spherical membrane bound structures) that contain within them water and neurotransmitter. The neurotransmitter is concentrated within the synaptic vesicles, so that in case of the NMJ, there might be 5000 molecules of “astocolli”? (check iona’s lecture) in each synaptic vesicle. The synaptic vesicle is capable of fusing with the presynaptic (beginning of the synapse) membrane. When the vesicles fuse with the membrane, they open a little from which the neurotransmitter can diffuse from where its most concentrated to the less concentrated space of the synaptic cleft. The cleft is just a short space between the nerve terminal and the postsynaptic cell. The neurotransmitter then has a structure that can bind to and recognise a receptor molecule because of its particular shape. When it binds to the receptor, it’ll activate the receptor and causes a signal in the postsynaptic cell. There’s fast and slow parts to this fast synaptic transmission, the fast part (parts that happen in less than a millisecond) of the depolarisation reaching the nerve terminal propagating down (i.e. saltatory conduction) depolarising the nerve terminal. The opening of the voltage gated calcium channels (inward diffusion of calcium) rapidly diffuses in as soon as the channel is open. The exocytosis of the neurotransmitter containing vesicles occurs very rapidly too (maybe 50microseconds). The postsynaptic polarisation i.e. hyperpolarisation will also occur very fast. The slower part, mainly to do with recovery so the neurotransmitter that is released has to be recaptured / “remanufactured”, so parts of the neurotransmitter are recycled or endocytosed by nerve terminals, recycled so they can be reused again. Then there’s the priming of synaptic vesicles for another round of transmission i.e. “rescuing” the membrane from the synaptic vesicle, it has to be refilled with neurotransmitter and prepared to release its contents again. There is a process called Neuromodulation that occurs at some but not all synapses (later). i.e. FAST: (<1 millisecond) Presynaptic depolarisation Inward Ca2+ current Exocytosis of neurotransmitter from primed synaptic vesicles Postsynaptic potential SLOW: (seconds) Recycling of neurotransmitter and synaptic vesicles Priming of synaptic vesicles ready for the next period of active of synaptic transmission Neuromodulation (mediated by metabotropic receptors) - - - - Typical nervous system synapse might look something like this, e.g. the synaptic input for the dendrites of the motor neuronthat receives excitatory inputs. Glutamate is released by those nerve terminals and it excites the motor neuron. E.g. bringing feedback information like an afferent (1a) bringing back information about the stretch of the muscle to excite the motor neuron. It contains many synaptic vesicles and when the action potential comes down through the nerve terminal it opens voltage gated calcium channels, the calcium can diffuse into the cell releasing neurotransmitters. The above is showing the recapture of the neurotransmitter, the neurotransmitter can either be taken up in the blood then pumped around the body and recycled in that way or in the case of glutamate, more likely, it will be pumped back into a glial cell. The CNS, those glial cells, that will be doing this job right next to the nerve terminal will be the astrocytes. This astrocyte glial cell will have a secondary active transporter that uses the concentration gradient of sodium to drag along with it a glutamate molecule into the glial cell. It pumps the glutamate out of the synaptic cleft and so, after the transmission has occurred, the glutamate represented by the red dots are removed largely by being pumped into the glial cells. In some cases the neurotransmitter may be broken down by an enzyme so that its no longer active. The important thing about removing the transmitter is that it doesn’t keep activating the receptors on the postsynaptic cell. Situations such as excitotoxic damage in central neurons e.g. following a stroke or some other kind of damage to the brain, it can get too glutamate inside synaptic clefts and causes damage to the postsynaptic cell and can cause neurons to die, which is assoicated with the kind of damage that can happen following stroke. These transporters are important for removing glutamate so that it only has a brief period of action before its removed from the synaptic cleft. i.e. Typical excitatory central synapse (synapse formed by a neuron on another brain neuron): • Releases only small amounts of neurotransmitter glutamate in response to presynaptic action potential • Glutamate opens cation channels (ligand-gated cation channels where glutamate is the ligand) on postsynaptic membrane • Depolarisation is small (1mV) as measured in the soma • Simultaneous activity of many synapses needed to bring postsynaptic neuron to threshold • Postsynaptic membrane may also contain metabotropic glutamate receptors that act via second messengers for neuromodulation of synaptic function - - - - In the CNS, the above blobby things are referred to as boutons (sometimes known as synaptic knobs). In the motor neuron, the depolarisation produced by the activity of each of these synapses add together a large amount of these excitatory glutamatergic nerve terminals i.e. ther boutons, the above represents one of them. Each one of them is capable of releasing glutamate (the neurotransmitter) which activates glutamate receptors on the postsynaptic cell which is on the dendrites. On the dendrites of the cells of the motor neurons of the brain and spinal cord, there are “spines”, these raised mushroom shaped things stick out from the dendrite and they have on their head receptors of the neurotransmitters. So the above would be a glutamatergic spine syanpse on a motor neuron. But there are large amounts of these on the motor neuron, they’re not all active on the motor neuron all the time, most of them are inactive most of the time. When enough of them are active they each produce local circuit currents, which spread down to the cell soma and can be depolarised. This one here may reach half a millivolt, not enough to reach threshold but if there is another there activated near the same time, then its local circuit current will add together with the previous one to produce a bigger depolarisation. If there are enough, it may reach threshold and the Hodgkin cycle would get started here. That’s how motor neurons are thought to work, by summing up all of the excitatory synaptic input coming down from the dendritic tree and concentrating on the cell soma and axon hillock. If it reaches threshold, it fires off a chain of action potentials. If it doesn’t reach threshold, there is nothing, i.e. the gating function of the neuron, only letting through signals that reach a certain threshold level. - - So when measuring membrane potential, single EPSP’s (excitatory postsynaptic potentials) due to one of the synapses being active are normally not sufficient to reach threshold however when two occur one shortly after the other, they sum together to possibly push the membrane past the threshold. That gets the voltage gated sodium channels going, the Hodgkin cycle and membrane depolarises. Those excitatory synapses are usually the opening of ligand gated cation channels. EPSC (excitatory postsynaptic currents) are the inward moving sodium ions that enter the postsynaptic cell when ion channels open on their membrane. They’re cation channels, i.e. permeable to potassium and sodium. Sodium diffuses when these channels are open at the synapse and that’s what causes the depolarisation we can measure as the EPSP and the current that’s moving through them is called ESPC. i.e. Synaptic integration in a motor neuron: • Plasma membrane stores electrical charge this means brief opening of channels results in much longer slower changes in Vm (capacitance properties) • Summation of EPSCs occurring within a few milliseconds of each other sum to raise V m • When Inhibitory Postsynaptic Currents (IPSCs) occur at the same time as EPSCs they help to lower the V m and reduce the chance that action potential/s will be triggered at the axon hillock Vast range of the synapses in the brain and spinal cord and in the periphery: - There are many different neurotransmitters (mostly small molecules), some of the important ones are either amino acids themselves or molecules very closely related to amino acids such as glutamate, glycine, acetylcholine (Ach), Gamaaminobutyric acid (GABA), Serotonin/5-hydroxytryptamine (5-HT), Dopamine, Noradrenaline/norepinephrine, ATP, etc. - Why we have all these different ones are because these can bind to different kinds of receptors and put some specificity in a way that one cell can talk to another cell. - For each neurotransmitter, there is at least receptor molecule on the post-synaptic side. - Most of them, there’s more than one e.g. - ACh to nicotinic receptor which is a ligand gated cation channel or muscarinic receptor, so when ACh is released onto smooth muscle cells, it activates a muscarinic type receptor and second messenger type signal. Specific receptors and their neurotransmitters won’t be examined however, be aware of them. HOWEVER, he will ask about ones he’s been talking about so most probably Glutamate. Be aware =) Why would we have all these different transmitter systems and, transmitters and receptors? - One of the reasons is that the signal that is coming down the nerve terminal, all axons, the signal passes down the action potentials and the excitatory signal raises the membrane potential. Sometimes, we need to reverse the signal and make it a negative signal, so translate a positive depolarisation into a negative hyperpolarisation, and the target cell i.e. postsynaptic cell, a neurotransmitter can be released to activate a different kind of ion channel, a ligand gated chlorine channel. Where will this come in? - - - From the motor neuron above, it receives excitatory inputs, it also receives many inhibitory synaptic inputs. These are just as important as the excitatory inputs in controlling when the motor neuron will fire and for how long. These inhibitory synapses basically work the same way, the action potential comes down, it releases a neurotransmitter which acts on a ligand gated ion channel on the postsynaptic cell, but this time the opening of these ligand gated ion channels causes the membrane potential not to go up but go down and become more negative. The neurotransmitters are typically GABAA or glycine and in the spinal cord when talking about motor neurons is the glycine neurotransmitter. The GABAA receptors and glycine receptors are ligand gated channels selective for Cl ions. The ligand gated Chloride channel (ligand is either the GABA in the case of the GABAA receptor or glycine in the case of the glycine receptor). The protein molecule that the receptor is composed of contains a channel. The channel is selectively permeable to chloride ions. Sodium and potassium can’t pass through however chloride can. These chloride channels have a different effect on the post synaptic cell. In an adult, there is a high concentration of chloride in the blood, but a low concentration in the cell body i.e. in the cytoplasm of the neuron. There is a strong chemical driving force driving chloride ions from the extracellular fluid into the cytoplasm. Chloride will enter the neuron when given the opportunity through these channels and it’s a negative ion so it will drive the membrane potential down. It will drive closer to the Nernst potential for chloride down around -70mV. The opening of ligand gated chloride channels and inhibitory synapses will have the effect of pulling the membrane potential down to Nernst’s potential and holding it down. The important thing about inhibitory synapses that work via chloride channels are that they make it harder for the membrane to reach threshold to trigger action potentials in the cells in which they’re located i.e. amps down the activity of neurons. i.e How do fast inhibitory synapses work? • GABAA receptors and glycine receptors are Ligand gated channels selective for Cl• [Cl-]o >>[Cl-]i concentration gradient into cell • Opening of these channels → ↑inward current of Cl equivalent to an ↑ outward current of +ve ions • Hyperpolarise soma, or short-circuit depolarising local circuit currents coming from excitatory synapses Fast and slow chemical synaptic transmission: FAST SYNAPTIC TRANSMISSION Transmitter binds to and opens ligand-gated ion channels, could be a ligand gated cation channel which will produce excitation because sodium will diffuse in or it could be a ligand gated chloride channel where there is an inhibitory synapse because it allows chloride ions which are negative, into the cell bringing the membrane potential down towards nert’s potential of -70mV. - In each case increasing the permeability of the ion, takes the membrane potential to nert’s potential for that ion. That happens really fast, as soon as the channels open very fast, and the ions will diffuse immediately in nanoseconds. - Response takes no more than a few milliseconds - “Inward and Outward” currents add up/summate SLOW SYNAPTIC TRANSMISSION/ NEUROMODULATION – as a result of indirect signalling mechanism. - Neurotransmitter/Neuromodulator binds to receptor that doesn’t contain an ion channel but which generates / activates a second messenger inside the cytoplasm of the targets that in turn may open a potassium channel for example, one that may be regulated by a second messenger. - These slow synaptic transmissions can go on in parallel with fast transmissions, importantly, a longer term effect it has is that it makes the neuron more excitable (more likely to fire off next time activated by synaptic inputs) or less excitable. - i.e. Regulates the excitability of the nervous system and important for changing things like the awareness state – whether or not were alert or awake. - Response takes seconds or minutes - Modulates excitability Neuromodulators • In addition to their roles in fast synaptic transmission, some chemicals released by nerve terminals can act as neuromodulators • Some neuromodulators act via receptors on the post-synaptic cell making the cell more or less excitable (likely to fire action potentials) • Some act via receptors on nerve terminals modifying the likelihood that transmitter will be released