Answer Key
advertisement

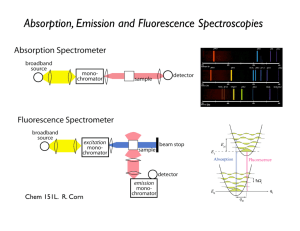
CHEM106: Assignment 12 Electronic Transitions 1. Which molecular processes correspond to absorption of microwave, infrared, ultraviolet-visible photons? Rotational excitation microwave Vibrational excitation IR Electronic excitation UV-Vis 2. Electronic transitions of a molecule can be probed by both absorption and emission spectroscopy. A. Draw a diagram to illustrate the basic setup of the experimental apparatus for the electronic absorption spectroscopy and fluorescence spectroscopy, respectively. a. Absorption Spectroscopy b. Fluorescence Spectroscopy B. Explain why the fluorescence spectroscopy is more sensitive than the absorption spectroscopy. The key difference is that fluorescence spectroscopy measures the intensity of fluorescence perpendicular to the direction of incident beam, whereas absorption spectroscopy measures the ratio of the intensities of two beams (incident and passed beams). The fluorescence signal is directly proportional to the intensity of the excitation source. Increase in the intensity of the excitation beam leads to a subsequent increase in the fluorescence signal, resulting in greater sensitivity. On the other hand, absorption depends on the ratio of incident (I0) to passed light (I). So simply increasing I0 also increases I (see above figure). 3. Define the FranckCondon principle, and illustrate it with a diagram. According to the Franck–Condon principle, an electronic transition is most likely to occur without changes in the positions of the nuclei in the molecular entity and its environment. Thus, the electronic transition is a vertical transition. The quantum mechanical formulation of this principle is that the intensity of a vibronic transition is proportional to the square of the overlap integral between the vibrational wavefunctions of the two states that are involved in the transition. As illustrated in the following diagram, the FranckCondon principle dictates that the transition from v’’ = 0 to v’ = 2 is the most intense for the absorption spectrum, and the transition from v’ = 0 to v’’ = 2 is the most intense for the emission spectrum. 4. The carbonfluorine bond in tetrafluoromethane (CF4) is one of the strongest in organic chemistry, with a bond dissociation energy of 113.0 kcal/mol. Calculate the maximum wavelength of light capable of breaking the CF bond. First, we convert E from kcal/mol to J/molecule. E 113.0 kcal 4184 J mol 7.857 10 19 J / molecule . mol kcal 6.02 10 23 molecules According to the Einstein equation, E h hc / . Solve for , and we have hc / E (6.626 10 34 Js )( 3 108 m / s ) 2.531 10 7 m 253.1 nm . 7.857 10 19 J Thus, it takes a photon in the UV region to break a CF bond. 5. What is the difference between a singlet (S) state and a triplet (T) state? What is the selection rule that governs the electronic transitions among S states and T states? A singlet state is a many-electron state in which all electron spins are paired. Total spin angular momentum for the singlet state is zero (S = 0), yielding a spin multiplicity of 2S + 1 = 1. This is commonly the multiplicity of neutral molecules in the ground state. A triplet state is a many-electron state in which two electron spins are parallel. Total spin angular momentum for the triplet state is 1 (S = 1), yielding a spin multiplicity of 2S +1 = 3. According to the spin selection rule for electronic transitions, S 0 . This means that singlet–singlet transition is spin allowed, and singlet–triplet transition is spin forbidden. 6. Consider a molecule that can fluoresce from the S1 state and phosphoresce from the T1 state. Draw an energy diagram representing absorbance, fluorescence, and phosphorescence.