Absorption, Emission and Fluorescence Spectroscopies
advertisement

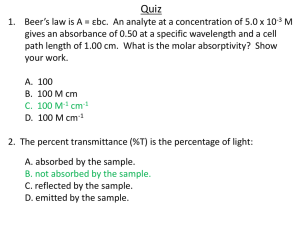
Absorption, Emission and Fluorescence Spectroscopies Chem 151L. R. Corn Review: Light behaves like an electromagnetic wave... 2.998 x 108 meters/sec λ = the Greek letter lambda ν = the Greek letter nu velocity = wavelength x frequency meters/sec = (meters) x (sec)-1 The units (sec)-1 are called Hertz (Hz)! Heinrich Hertz 1857-1894 Review: But light also behaves like a particle! We call the particles “photons”, each with a energy given by: h = Planck's constant 6.626 × 10−34 Joule sec Max Planck 1858-1947 Review: Atoms have quantized energy levels too, and can absorb and emit photons of energy equal to the difference in energy between two levels ∆E = hν Grotrian diagram for Hydrogen Review: Molecules also have quantized energy levels, and can absorb and emit photons of energy equal to the difference in energy between two levels: LUMO hν HOMO M + hν → M* ∆E = hν Absorption Spectroscopy measures the amount of light absorbed by a sample by this process. M + hν → M* Transmittance: T = P/P0 Absorbance: A = log(P0/P) For atoms: Atomic Absorption Spectroscopy For molecules: Molecular Absorption Spectroscopy The Absorption Spectrum plots the amount of light absorbed by a sample as a function of photon wavelength. Absorption spectrum Beer’s Law relates the amount of light absorbed by a sample to the concentration of the absorbing species. Absorbance A = log(P0/P) Beer’s Law: A = εbc b = pathlength (in cm) c = concentration (in M) ε = molar absorptivity (units of M-1cm-1) Absorption versus Emission Absorption is the process that consumes a photon and puts the atom or molecule in an excited state. M + hν → M* Emission is the process that creates a photon and takes the the atom or molecule in an excited state back to the ground state. M* → M + hν The Emission Spectra of H, He and Hg. Fluorescence Spectroscopy Fluorescence is the process that first consumes a photon and puts the atom or molecule in an excited state... M + hν → M* And then emits a photon of lower energy which takes the the atom or molecule back to the ground state. M* → M + hν’ hν > hν’ Fluorescence Spectroscopy Net reaction: M + hν → M* → M + hν’ hν > hν’ The emitted photon has less energy than the absorbed photon because the molecule loses some energy (by vibrating and rotating) in the excited state: M* M hν’ The Fluorescence Spectrum plots the amount of light absorbed by a sample as a function of photon wavelength. P0 P PF P0 = power of incident light beam (units: W) P = power of transmitted light beam. PF = power of emitted fluorescence. Quantitative Fluorescence Spectroscopy The power of the emitted fluorescence is proportional to the absorbed power, P0-P: PF = ΦF (P0 − P) where ΦF, the quantum yield, is: € photons emitted ΦF = photons absorbed substituting from Beer’s law: € ( PF = ΦF P0 1− e −εbC ) Quantitative Fluorescence Spectroscopy ( PF = ΦF P0 1− e −εbC ) the exponential here can be expanded as follows: n ⎞ ⎛ εbC (εbC) 2 (εbC) € PF = ΦF P0εbC⎜1− + − ... ⎟ 2! 3! (n + 1)!⎠ ⎝ and just the first term here is significant for small εbC: PF = ΦF P0εbC PF is proportional to concentration at small εbC. Fluorescence Spectroscopy The absorption and fluorescence spectra of riboflavin. absorption riboflavin fluorescence