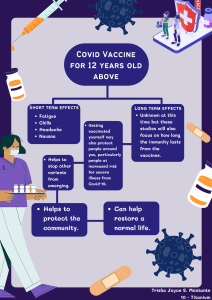
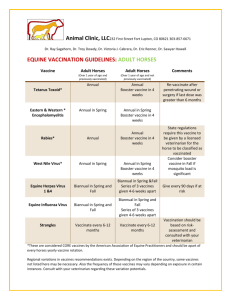
Vaccines Input Form
advertisement

Form for input on priorities for research and development for vaccines for important Transboundary Animal Diseases for the National Veterinary Stockpile. Please fill out one form for each disease. Return the forms via email to iicab@iastate.edu by Friday, September 21, 2012. Name (optional):___________________________________ Role in meeting: _______Member of scientific advisory committee _______Speaker _______Participant _______Academia _______Industry _______Federal Agency _______Other:_________________ Recommendations for______________________________________: Rift Valley Fever HPAI Exotic Newcastle disease FMD Nipah and Hendra African Swine Fever Classical Swine Fever Schmallenberg Virus Q Fever Heartwater Ebola Please indicate the level of importance for the following characteristics for an emergency use vaccine for this disease: H = High Priority M = Medium priority L = Low priority NA = Not applicable for a vaccine for this disease Leave blank = I don’t know ______Rapid onset of immunity ______Long duration of immunity ______Safe for manufacturing under BL2 conditions in a disease free country ______Broad protection for multiple strains or serotypes. Induction of heterosubtypic immunity Induction of: o ______Serum neutralizing antibody o ______TH 1 cytokine response o ______Cytotoxic T cells o ______Mucosal IgA o ______T cell mediated immunity at mucosal surface Desirable characteristics for stockpiling for emergency use o ______Ongoing manufacture and sale in endemic countries which enables indefinite delivery/indefinite quantity (IDIQ) contracts for just in time delivery o ______Stable when stored as bulk antigen o ______Long self-life of finished vaccine o ______Likelihood that it will protect against future strains/serotypes of pathogen ______Companion diagnostic test to detect infection in vaccinated animal (DIVA) ______Positive marker to detect vaccinated animals ______Capability to meet 9 CFR regulatory requirements for purity, potency, safety, and efficacy ______Capability to meet requirements for cost effective manufacturing and all proprietary rights to vaccine antigen, vectors, and/or adjuvants ______Safe for use in food producing animals with no, or reasonably short, withdrawal time for animal products for human consumption ______Not hazardous for humans accidentally exposed to the vaccine ______Suitable for mass vaccination strategies (eg. Oral delivery) ______Safety and efficacy in multiple species affected by the pathogen (list species) ______Vaccine capable of being delivered to wildlife (eg. In baits) ______Knowledge of level of herd immunity needed to stop transmission in a population (Reproduction ratio) ______Thermostability to maintain efficacy in absence of a reliable cold chain ______Compatible with World Organization for Animal Health (OIE) requirements for safety and efficacy of vaccines for international trade ______Other: What are the best near term opportunities for developing a vaccine for the NVS? What are the major deficiencies of current vaccines and vaccines under development? Are there major research questions which need to be answered before vaccines (or improved vaccines) can be developed for this disease? If so, what are they? What technology shows the greatest promise for long term development of a next generation vaccine for the NVS? Other Comments: