Media Release
advertisement

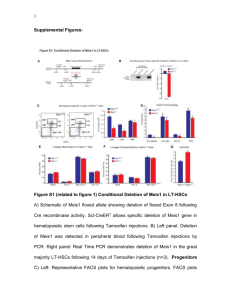
Boosting the ability of the urinary tract to fight infections EMBARGO: April 30, 2015, 11am PDT / 2pm EDT Urinary tract infections (UTIs) are common, and wide-spread antibiotic resistance has led to urgent calls for new ways to combat these infections. A study published on April 30th in PLOS Pathogens reports that an experimental drug that stabilizes the human immune defense protein HIF-1α can protect human bladder cells and mice against a major UTI pathogen, and it might provide a therapeutic alternative or complement to antibiotic treatment. HIF-1α is known to play a key role in modulating the innate (non-specific) immune response, which is the body’s first line of defense against intruding pathogens. Like many regulator proteins, HIF-1α is relatively short-lived. To increase HIF-1α levels, researchers have developed drugs that delay its break-down. Such HIF-1α stabilizers are in clinical development for treatment of anemia, and in this study Victor Nizet, from the University of California, San Diego, USA, and colleagues explored the potential use of these drugs as “innate immune boosters” against uropathogenic E.coli (UPEC) bacteria that are a major cause of UTIs. In human urinary tract cells, treatment with the drugs indeed increased HIF-1α levels in healthy cells. Such cells were then more resistant to UPEC attachment, as well as subsequent invasion and killing by the bacteria, than human urinary tract cells with normal HIF-1α levels. Using an established mouse UTI model, the researchers showed that administration of HIF1α stabilizers directly into the bladder protected the mice against UPEC infection of the bladder and kidney. They also found that invasion of bladder cells, a critical early step in the infection process, was reduced in treated mice compared to untreated ones. To verify the importance of HIF-1α in the defense against UPEC infection, the researchers studied mutant mice with much reduced HIF-1α levels. Exposed to UPEC, these mice were more susceptible to bladder infection, and pre-treatment with HIF-1α stabilizers made no difference. This demonstrates that the drugs attenuate UTIs through their effect on HIF1α. Finally, the researchers examined whether treatment with HIF-1α stabilizers would be beneficial even against an established UTI. To do this, they infected mice with UPEC first and then administered the drugs into the bladder 6 hours later. The treated mice had a more than 10-fold reduced rate of bladder colonization, demonstrating that HIF-1α stabilization is beneficial even after the initial infection. The researchers conclude that their “combined data indicate that by stabilizing HIF-1α, AKB-4924 [the specific HIF-1α stabilizer they used] enhances production of antimicrobial effectors in both prophylactic and therapeutic settings, promoting bacterial clearance, and suggesting HIF-1α boosting as a potential adjunctive therapeutic strategy in UTI management.” The next steps include testing HIF-1α stabilizers in a clinical trial setting in humans, and making versions of the drug that can be taken orally and reach the urinary tract. Please use this URL to provide readers access to the paper (Link goes live upon article publication): http://dx.plos.org/10.1371/journal. ppat.1004818 Press-Only Preview Of The Article: https://www.plos.org/wp-content/uploads/2013/05/PATHOGENS_NIZET_APR30.pdf Related Image for Press Use: https://www.plos.org/wp-content/uploads/2013/05/PATHOGENS_NIZET_APR30_IMG.tif Caption: Compared with UPEC-infected control mouse bladders (vehicle), bladders treated with the HIF-1alpha stabilizer (AKB-4924) express higher levels of the defense molecule CRAMP (red). Credit: Nizet et al., CC-BY Contact: Victor Nizet e-mail: vnizet@ucsd.edu phone: +1.858.534.7408 Authors and Affiliations: Ann E. Lin, UC San Diego, USA Federico C. Beasley, UC San Diego, USA Joshua Olson, UC San Diego, USA Nadia Keller, UC San Diego, USA Robert A. Shalwitz, Aerpio Therapeutics, USA Thomas J. Hannan, Washington University School of Medicine, USA Scott J. Hultgren, Washington University School of Medicine, USA Victor Nizet, UC San Diego, USA Please contact plospathogens@plos.org if you would like more information. Funding: The research was supported by NIH grants to VN (AI093451, AI057153, HD071600) and SJH (DK098870, AI048689). AEL was supported by postdoctoral fellowships from the Canadian Institute of Health Research and the American Association of Anatomists. RAS was an employee of Aerpio Therapeutics and received salary support from the company. None of the grant funders nor Aerpio Therapeutics had a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: RAS is an employee of Aerpio Therapeutics, which has sought to develop AKB-4924 for its immunomodulatory properties in inflammatory bowel disease. This does not alter our adherence to all PLOS policies on sharing data and materials. Citation: Lin AE, Beasley FC, Olson J, Keller N, Shalwitz RA, Hannan TJ, et al. (2015) Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Escherichia coli Infection. PLoS Pathog 11(4): e1004818. doi:10.1371/journal.ppat.1004818 About PLOS Pathogens PLOS Pathogens is a peer-reviewed, open-access science journal that advances the understanding of bacteria, fungi, parasites, prions, and viruses, and how these pathogens interact with their host organisms. For more information, visit http://www.plospathogens.org and follow @PLOSPathogens on Twitter. About the Public Library of Science PLOS is a nonprofit publisher and advocacy organization founded to accelerate progress in science and medicine by leading a transformation in research communication.