file - Reproductive Health
advertisement

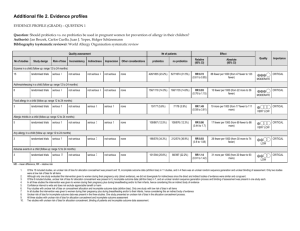
Additional file 3: Risk of bias within studies 1) Risk of bias assessment for randomized controlled trials based on the Cochrane Collaboration’s risk of bias criteria Extremely preterm birth (RCTs) 1. Study ID Type of study Bias Sequence generation Allocation concealment Blinding of participants and personnel Blinding of outcomes Incomplete outcome data 2. Support for judgment High Not blinded Unclear Low Not mentioned All patients completed study All specified outcomes in methods reported following the standard protocol Insufficient information to assess other bias exsit Selective reporting Low Other bias Study ID Type of study Unclear Romero 2000 RCT Author’s judgment Low Low Bias Sequence generation Allocation concealment Blinding of participants and personnel Blinding of outcomes Incomplete outcome data Selective reporting Other bias 3. Richter 2005 RCT Author’s judgment Unclear Unclear Study ID Type of study Bias Sequence generation Allocation concealment Blinding of participants and personnel Blinding of outcomes Incomplete outcome data Selective reporting Other bias Low Not described Not described Support for judgment Computer-generated randomization Opaque envelopes Double blinded Low Low Low Blinded Intended to treat; same numbers, same reasons for attrition Study protocol available and same outcomes reported Imbalance of baseline characteristics, with younger preterm Unclear births allocated to atosiban group The Canadian Preterm Labor Investigators Group (PLIG) 1992 RCT Author’s Support for judgment judgment Patients, physicians and nurses blinded to women’s treatment Low allocation Low Done by pharmacy–controlled randomisation Low Double blinded Low Unclear Low Low Blinded Loss to follow-up unclear, not explained in detail Study protocol available and all expected outcomes reported The study appears to be free of other sources of bias 2) Risk of bias summary for studies included extremely preterm birth (RCTs) 3) Risk of bias assessment for non-randomized studies based on the Risk of Bias Assessment tool for Non-randomized Studies’ criteria (RoBANS) Extremely preterm birth (non-RCTs) 1) Study ID Type of study Bias 2) Selection of participants Confounding variables Measurement of exposure Blinding of outcomes Incomplete outcome data Selective reporting Study ID Type of study Bias Selection of participants Confounding variables Measurement of exposure 3) Blinding of outcomes Incomplete outcome data Selective reporting Study ID Type of study Bias Selection of participants 4) Confounding variables Measurement of exposure Blinding of outcomes Incomplete outcome data Selective reporting Study ID Type of study Bias Selection of participants Confounding variables Measurement of exposure Blinding of outcomes Incomplete outcome data Selective reporting Berghella 2009 Retrospective cohorts Author’s Support for judgment judgment Low All women with high risk preterm select from medical database Low Confounding variables adjusted by multivariable regression Low Medical examination Low Not blinded, but outcome most likely not influenced Unclear The number of excluded or missing data were not described Low Results correspond to intended estimate outcomes reported Cape 2010 Retrospective cohorts Author’s Support for judgment judgment Unclear Population’s origin not described Low Adjusted through Cox regression Although source is unclear, information was only found in Unclear medical records Low Not blinded, but outcome most likely not influenced Unclear Not mentioned clearly Unclear Abstract report contained too few information Manuck 2012 Retrospective cohort Author’s Support for judgment judgment All women with high risk preterm select from a single Low healthcare system database High Not adjusted Low Medical records Low Not blinded, but outcome most likely not influenced Unclear Not mentioned Unclear Abstract report contained too few information Visintine 2008 Retrospective cohort Author’s Support for judgment judgment All women in the study have same risk and in same University Low database Low Confounding variables adjusted by logistic regression Low Medical records Low Not blinded, but outcome most likely not influenced Low Identified data and loss to follow-up described High Side effects and adverse outcomes not assessed 4) Risk of bias summary for studies included extremely preterm birth (non-RCTs)