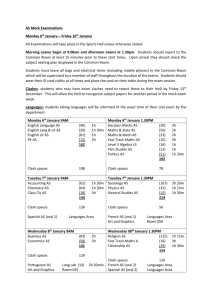
Organic Chemistry PBL Exam Questions
advertisement
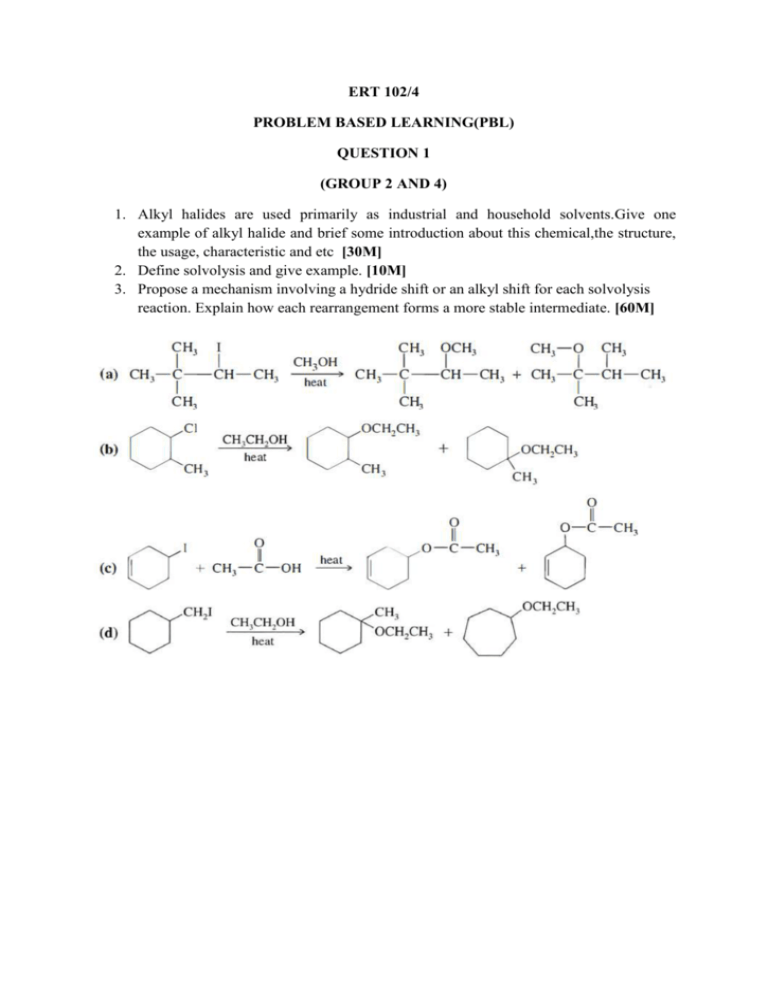
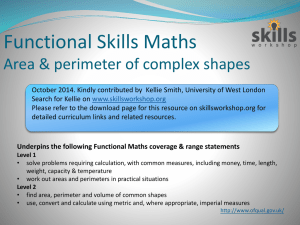
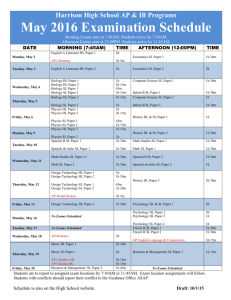
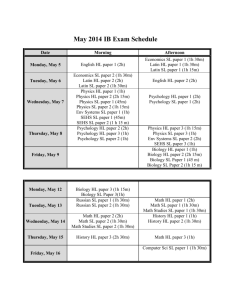
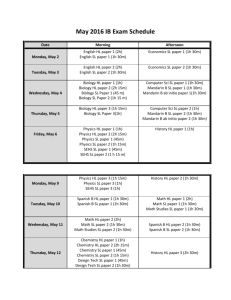
ERT 102/4 PROBLEM BASED LEARNING(PBL) QUESTION 1 (GROUP 2 AND 4) 1. Alkyl halides are used primarily as industrial and household solvents.Give one example of alkyl halide and brief some introduction about this chemical,the structure, the usage, characteristic and etc [30M] 2. Define solvolysis and give example. [10M] 3. Propose a mechanism involving a hydride shift or an alkyl shift for each solvolysis reaction. Explain how each rearrangement forms a more stable intermediate. [60M] QUESTION 2 (GROUP 1 AND 3) 1. Bisphenol A is an important component of many polymers, including polycarbonates, polyurethanes, and epoxy resins. a) Give some introduction about this chemical,the structure, the usage, characteristic and etc. (30 M) b) Bisphenol A were synthesized from phenol and acetone with HCL as a catalyst. Propose a mechanism for this reaction. (50M) 2. Identify A to I in the reaction below and give the iupac name for the last product (20M); QUESTION 3 (GROUP 5 AND 7) 1. Cyclohexanol is the organic compound with the formula (CH2)5CHOH. This compound exists as a deliquescent colorless solid, which, when very pure, melts near room temperature. a) Give some introduction about this chemical,the structure, the usage, characteristic and etc. [30M] b) Show how you would synthesize the following compounds. As starting materials, you may use cyclohexanol and any necessary solvents and inorganic reagents. [20M] 2. Two products are observed in the following reaction. (a) Suggest a mechanism to explain how these two products are formed.[30M] (b) Your mechanism for part (a) should be different from the usual mechanism of the reaction of SOCl2 with alcohols. Explain why the reaction follows a different mechanism in this case.[20M] QUESTION 4 (GROUP 6 AND 8) 1) Benzene is an aromatic hydrocarbon which are colorless, flammable liquid with a sweet odor. Give some introduction about this chemical,the structure, the usage, characteristic and etc. [30M] 2) The compound shown reacts with HBr to give a product of molecular formula C10H11Br. a) Propose a mechanism for this reaction and predict the structure of the product. Carefully show the resonance stabilization of the intermediate.[20M] b) When the reaction takes place in the presence of a free radical initiator,the product is a different isomer of formula C10H11Br. Propose a structure for this second product,and give a mechanism to account for its formation.[30M] 3) Give the structures of compounds A through H in the following series of reactions.[20M] Question 5 (Group 9 & 10) 1. Define the alkene and explain its physical properties. [20M] 2. Show the following transformation may be carried out. Include your retrosynthetic reasoning.[50M] 3. From the above reaction, identify which steps involved the E2 elimination and explain the factor why E2 elimination occurs in this reaction.[30M] Question 6 (Group 11 & 12) 1. Define the alkyne and explain its physical properties. [20M] 2. Although you might expect 1-propyne to be more reactive than 1-propene in electrophilic addition reactions, the reverse is true. Explain this using your knowledge of the mechanism of electrophilic additions to both alkynes and alkenes (include the structures in your explanation).[30M] 3. There are many reaction occur in our research lab and industries. Why and how the product can be formed? As good researcher you will design a good synthesis that involve many of the reactions. More over each step in the synthesis should provide the greatest possible yield of the desired product. Propose a synthesis that will achieved the following transformation. Be sure to show all reaction and intermediate products along the way. [50M] Acetlyne trans-2-methyl-octane Question 7 (Group 13 & 14) 1. Complete the following syntheses. For full credit, be sure to show all intermediary products, reagents, and solvents.[60M] (a) (b) 2. Which carbonyl is more susceptible to nucleophilic attack, that of cyclohexanone or that of hexanal? Provide two reasons for your choice.[25M] 3. Give three different sets of reagents (a carbonyl compound and a Grignard reagent) that could be used to prepare the following compound.[15M] Question 8 (Group 15) 1. For this pair of molecules, circle the stronger acid and provide a reason for your decision. Use pictures as well as words in your explanation.[30M] 2. For this pair of molecules, circle the stronger acid and provide a reason for your decision. Your reason should include pictures as well as words.[30m] 3. Provide two explanations for the following trend in reactivity. One of your explanations must use MO theory.[40M]