States of Matter Worksheet: Energy & Molecular Properties
advertisement
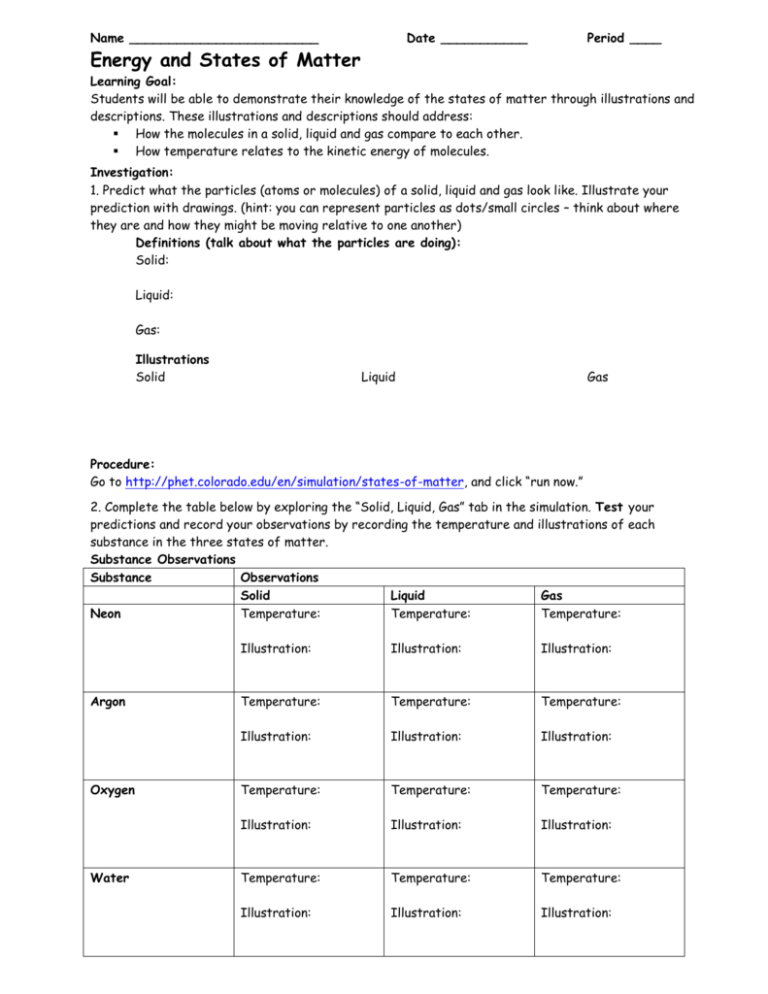
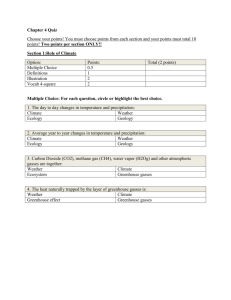
Name ________________________ Date ___________ Period ____ Energy and States of Matter Learning Goal: Students will be able to demonstrate their knowledge of the states of matter through illustrations and descriptions. These illustrations and descriptions should address: How the molecules in a solid, liquid and gas compare to each other. How temperature relates to the kinetic energy of molecules. Investigation: 1. Predict what the particles (atoms or molecules) of a solid, liquid and gas look like. Illustrate your prediction with drawings. (hint: you can represent particles as dots/small circles – think about where they are and how they might be moving relative to one another) Definitions (talk about what the particles are doing): Solid: Liquid: Gas: Illustrations Solid Liquid Gas Procedure: Go to http://phet.colorado.edu/en/simulation/states-of-matter, and click “run now.” 2. Complete the table below by exploring the “Solid, Liquid, Gas” tab in the simulation. Test your predictions and record your observations by recording the temperature and illustrations of each substance in the three states of matter. Substance Observations Substance Observations Solid Liquid Gas Neon Temperature: Temperature: Temperature: Argon Oxygen Water Illustration: Illustration: Illustration: Temperature: Temperature: Temperature: Illustration: Illustration: Illustration: Temperature: Temperature: Temperature: Illustration: Illustration: Illustration: Temperature: Temperature: Temperature: Illustration: Illustration: Illustration: 3. Look up the definition of Kinetic Energy. Write 1-2 sentences explaining how you think kinetic energy relates to temperature (i.e. If the kinetic energy increased, what would happen to the temperature? What if it decreased?) Kinetic Energy: How Kinetic Energy relates to Temperature: 4. Write a summary paragraph, which includes drawings, to demonstrate you have mastered the learning goal. Be sure to incorporate both concepts of the learning goal: How the molecules in a solid, liquid and gas compare to each other. How temperature relates to the kinetic energy of molecules. Temperature Scales We will primarily be using Celsius (˚C) and Kelvin (K) temperature scales in this class. We will briefly discuss Fahrenheit, but won’t use it much. One degree Celsius and one Kelvin are the same size, but they are offset by 273 degrees. The equations for converting between Celsius and Kelvin are below: K = ˚C + 273 ˚C = K – 273 Use the equations to convert the following temperatures: ˚C K 0 273 0 100 100 25 310