Propspective Trial COmparing Oxybutynin
advertisement

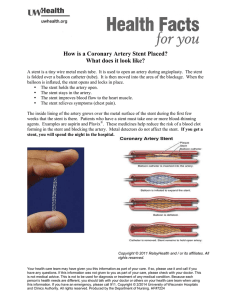
Phoenix Children’s Hospital Research Institute (PCRI) Website Clinical Trial Listing ** Please complete and return to Shy Walker at swalker@phoenixchildrens.com Study Title: Prospective Trial Comparing Oxybutynin and Tamsulosin for Stent Pain in the Pediatric Population Study Purpose The primary objective of this study is to evaluate the efficacy and safety tamsulosin in reducing stent discomfort in the pediatric population in the peri-operative period. The secondary objective is to compare the efficacy of tamsulosin with that of oxybutynin in reducing stent discomfort. Study Summary Primary Outcome Measures: Number of doses of pain medication used. After surgery, each child will be prescribed the standard dosage of oxycodone/acetaminophen based on their weight. We will have the patient's family record the number of doses of narcotics used from postoperative day #1 to post-operative day #5 to assess their pain needs. Pain scale based on the faces pain score We will have the family members record the child's pain score in the morning and evening using the FACES pain scale and record the score. Basic Eligibility Criteria Inclusion Criteria: Patients aged 4-18 years will be enrolled in this study. We will include all patients who will have a ureteral stent placement after their procedure. Exclusion Criteria: Patients with developmental delay or unable to verbalize their pain level will be excluded. Study Location(s): Phoenix Children’s Hospital Study Contact(s): Michael Nguyen, MD Catherine Chen, MD Division of Urology