Applied Physics Letters
advertisement

Supplementary Materials
Effects of core/shell structure on magnetic induction heating promotion
in Fe3O4/γ-Fe2O3 magnetic nanoparticles for hyperthermia
Shih-Chi Lee,1 Chao-Ming Fu,1,a) and Fu-Hsiung Chang2
1
Department of Physics, National Taiwan University, Taipei 10617, Taiwan
2
Institute of Biochemistry and Molecular Biology, National Taiwan University, Taipei 10051, Taiwan
Determination of Fe3O4/γ-Fe2O3 core-shell MNPs
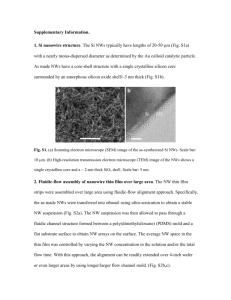
For the confirmation of Fe3O4/γ-Fe2O3 core-shell MNPs, ESCA was utilized to examine the
characteristic peaks of MNPs to identify the chemical composition, as shown in Fig. S1.
Particularly, the O 1s spectrum indicates the information containing the presence of oxygen (e.g.,
oxide, hydroxide, and physically-adsorbed water)1 on their surface and/or inside the material, as
shown in Fig. S1(a). Figure S1(b) shows that the features of MNPs fit in with the characteristics
of magnetite at peaks of about 710.2 eV and 723.8 eV corresponding to the spin-orbit split
doublet of Fe 2p1/2 and Fe 2p3/2, respectively. In addition, the binding energy of spin-orbital
splitting between Fe 2p1/2 and Fe 2p3/2 is about 13.6 eV for magnetite as well. However, the
satellite line, situated at about 718.5 eV, determines the existence of maghemite for MNPs.
Hence, these results strongly suggest that the crystalline phase is a mixture of both maghemite
and magnetite. Moreover, Fig. S1(c) shows XRD pattern of MNPs to indicate that those
characteristic Miller indices are almost dominated by spinel crystallinity of magnetite. 2,3
a)
Author to whom correspondence should be addressed. Electronic mail: chaomingfu@phys.ntu.edu.tw.
According to the aforementioned analyses, spinel maghemite should be conjugated on the
surface of Fe3O4-dominant MNPs. The core/shell structure of Fe3O4/γ-Fe2O3 MNPs were further
examined and demonstrated by high-resolution TEM (HRTEM; FEI Tecnai-G2 T20) comparing
the original and negative images as well, as shown in Fig. S1(d). The HRTEM image is taken at
TEM Laboratory, Department of Physics, National Taiwan University. The region with obvious
crystalline pattern is magnetite-core due to the contribution of magnetite-dominant crystalline
phase. According to the above, the outside region of core is maghemite for ultrathin shell.
FIG. S1. ESCA spectrum of MNPs in the (a) full, O 1s and (b) Fe 2p regions for determination of Fe3O4 and γ-Fe2O3
components. (c) Comparing XRD pattern of MNPs with JCPDS cards of Fe3O4 and γ-Fe2O3, and quantitative
analysis to both components of Fe3O4 and γ-Fe2O3 via pseudo-Voigt function. (d) Comparing the original and
negative images of HRTEM for structural profile of a Fe3O4/γ-Fe2O3 core-shell MNP.
Derivation of actual size distribution
According to the assumption of log-normal distribution,4 the size growing or diminishing rate
for a particle with size D can be expressed as
D D1 D2 D ,
dD
C
dt
D2 D1
(S1)
where D1 and D2 are the minimal and maximal size respectively, and C is a velocity
constant of formation. The normally distributed evolutionary time of growth or disappearance
(t ) exp{t 2 / 2 t2}/ t 2 is converted by (t )dt ( D)dD into the size distribution
( D) to obtain
2
1
D D1
D2 D1
1
D
exp 2 ln
,
2 D D1 D2 D
D2 D
2
(S2)
where is an equivalent polydispersity index of dimensional distribution, and is a
transitional constant related to the characteristic diameter D0 , i.e., the characteristic diameter is
the most probable size. By the extreme value theorem, we have
D0 D1
2 D2 D1 2 D0
.
D2 D1
D2 D0
ln
(S3)
Consequently, the actual size distribution would be derived from Eq. (S2) and Eq. (S3) to be
2
1 D D D D
D2 D1
1
2 D2 D1 2 D0
2
0
1
D
exp 2 ln
. (S4)
D2 D1
2 D D1 D2 D
2 D2 D D0 D1
When D1 and D2 tend to infinitesimal and infinite respectively, Eq. (S4) would become the
log-normal distribution (i.e., regular size distribution) as
1 D 2
1
D
exp 2 ln
.
D 2
2 D0
(S5)
Figure S2 shows the comparison with the actual and regular size distributions, any characteristic
diameter D0 can fit in with corresponding peak in the actual size distribution except the regular
size distribution. The actual size distribution should be able to accurately determine the
dimensional information of sample for a general case. From the fitting result of TEM histogram
in Fig. 1 for MNPs, the dimensional parameters were obtained the minimal grain diameter
D1 3.9 nm , maximal grain diameter D2 12.2 nm , characteristic grain diameter D0 7.0 nm ,
polydispersity index 0.9 , mean size of MNPs D 7.5 nm , standard deviation of size
distribution D 1.7 nm , mean magnetite-core diameter Dc 6.9 nm , standard deviation of
magnetite-core diameter Dc 1.6 nm , mean maghemite-shell thickness 0.3 nm and
standard deviation of maghemite-shell thickness 0.1 nm .
FIG. S2. Simulation to comparison with actual and regular distributions demonstrated by varying (a)
for their dissimilarities.
D0 and (b)
Analysis of thermal stability for Fe3O4/γ-Fe2O3 core-shell MNPs
The single-domain MNPs above the blocking temperature become superparamagnetism due
to the thermally susceptible fluctuation. The distribution of blocking temperature is giving by
f TB d TM ZFC T dT |T TB .5,6 Figure S3 shows zero-field cooling (ZFC) and field cooling
(FC) magnetization curves of core-shell MNPs measured from 10 K to 350 K in the applied
magnetic field of 100 Oe, which indicates that the core-shell MNPs exist two blocking
temperatures of about 131 K and 284 K. The high blocking temperature may be dominated by
the interfacial coupling between magnetite and maghemite. The low blocking temperature may
be ascribed to the thermal reversal of single domain in the small bulk magnetite and maghemite.
Hence, the core-shell MNPs would homogeneously rotate above 300 K in the presence of
alternating magnetic field.
FIG. S3. Magnetic properties of core-shell MNPs: ZFC/FC curves embedded with blocking temperature distribution
at static magnetic field of 100 Oe. The blocking temperatures were analyzed by the Gaussian-Lorentzian (GL)
mixture function.
Analysis of magnetism and heating characteristics for Fe3O4/γ-Fe2O3 core-shell MNPs
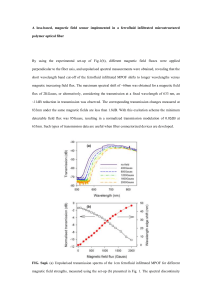
The hysteresis loops of core-shell MNPs were measured by individually applying magnetic
fields of 10 kOe, 100 Oe and 10 Oe at 300 K, as shown in Fig. S4(a)-S4(c). Regarding to
instrumental buffering rate and sampling settings, the rates of changing magnetic field for 10
kOe, 100 Oe and 10 Oe can be estimated as 0.50 Oe/sec, 0.25 Oe/sec and 0.05 Oe/sec
respectively. With increasing the rate of changing magnetic field, the coercivity of
rate-dependent hysteresis increases. Furthermore, the specific loss power SLP
cwater dT
mass dt
is
t 0
defined as the thermal power dissipation of MNPs, where cwater is about 4.185 J/gK, and
mass mMNPs / mwater is the mass fraction of MNPs in water. The experimental SLPs of core-shell
MNPs were measured by various amplitudes of alternating magnetic field with frequency of 89
kHz at 300 K, as shown in Fig. S4(d). According to the rate-dependent hysteresis model taken
the fitting result of size distribution into account, all experimental hysteresis loops and SLPs
were well fitted by the theoretical model. From the fitting result of hysteresis loops and SLPs, we
u
can obtain the saturation magnetization M s and magnetic interface anisotropy K int
of
core-shell MNPs to further analyze the influence on size-dependent coercivity and SLP in Fig. 2.
FIG. S4. Numerically fitting analysis to the hysteresis and magnetic induction heating experiments for core-shell
MNPs: the magnetic properties of hysteresis loops of size-distributed MNPs powder measured at static magnetic
fields of (a) 10 kOe, (b) 100 Oe and (c) 10 Oe to respectively simulate the hysteresis loops with various uniform
sizes for fitting. (d) Magnetic induction heating characteristics for MNPs suspension of 550 μL measured at various
alternating magnetic fields with frequency of 89 kHz.
REFERENCES
1
S. Yamamoto, H. Bluhm, K. Andersson, G. Ketteler, H. Ogasawara, M. Salmeron, and A.
Nilsson, J. Phys.: Condens. Matter 20, 184025 (2008).
2
T. Yamanaka and Y. Takéuchi, Z. Kristall. 165, 65 (1983).
3
W. Kim, C. Y. Suh, S. W. Cho, K. M. Roh, H. Kwon, K. Song, and I. J. Shon, Talanta 94, 348
(2012).
4
R. R. Irani, J. Phys. Chem. 63, 1603 (1959).
5
E. P. Wohlfarth, Phys. Lett. A 70, 489 (1979).
6
R. S. DiPietro, H. G. Johnson, S. P. Bennett, T. J. Nummy, L. H. Lewis, and D. Heiman, Appl.
Phys. Lett. 96, 222506 (2010).