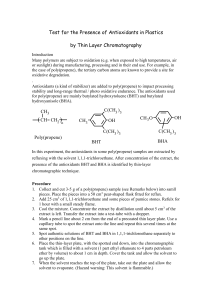
1. Introduction 1.1 Definition & Mechanism Antioxidants are
advertisement

1. Introduction 1.1 Definition & Mechanism Antioxidants are substances that protect cell components from being attacked by oxygen. The adverse effects of free radicals on normal physiological functions of cells will be decreased significantly with the presence of antioxidants in the body. Thus, cell damage from oxidation can be reduced or prevented. Oxygen will trigger the formation of free radicals in the body and this will cause chain reactions in cells that damage the cell structure and impair cell functions. Research found that these oxidative damages are linked to the development of many diseases such as Alzheimer’s disease, atherosclerosis and kidney diseases. Free radical damages Polyunsaturated fatty acid DNA & RNA Proteins Lipid radicals Altered DNA & RNA Altered proteins Impairing cell functions and eliciting inflammatory responses Cell damage, diseases and aging Diagram 1.1: Attack of free radicals in the cell components Diagram 1.2: Metal ion chelating mechanism by free radicals in the cells Diagram 1.3: Enzyme-catalyzed reaction of free radicals 2. Classification of antioxidants Antioxidants can be generally categorized into two broad divisions which are natural and synthetic antioxidants. Examples of natural antioxidants we will discuss are carotenoid (-carotene), ascorbic acid (vitamin C) and α-tocopherol (vitamin E) whereas examples for synthetic antioxidants are butylated hydroxyanisol (BHA) and butylated hydroxytoluene (BHT). 2.1 Natural antioxidants 2.1.1 Carotenoid (-carotene) Diagram 2.1: Structure of carotenoid, -carotene Carotenoids possess an extended system of conjugated double bonds that make them soluble in lipids and capable of quenching singlet molecular oxygen (1O2) and of free radicals. Because of this ability, carotenoids are thought to be protective against several diseases. Epidemiological studies have shown that people with high intake of fruits and vegetables, which are also rich in carotenoid have a lower incidence of diseases such as cardiovascular disease, cancer, cataracts and age-related macular degeneration. However, some intervention trials done in Finland show that there is no benefit in cancer prevention for people who has a long-term smoking habit (Gropper et al., 2009). They even show that intake of -carotene with vitamin A will further increase the risk of lung cancer and mortality from cancer and heart disease in the smokers. Contrarily, others suggest that the chance of getting chronic diseases and lung cancer will be increased only with the cofactors such as alcohol consumptions and long history of smoking. 2.1.2 Ascorbic acid (Vitamin C) Diagram 2.2: Structure of ascorbic acid, vitamin C Vitamin C is one of the important antioxidant that can protect the water-soluble substances from oxidizing agents. By being oxidized itself, it will regenerate already-oxidized substances to their original, active form. Some studies suggest vitamin C may reduce the oxidation of low density lipoprotein (LDL) and raise the level of high density lipoprotein (HDL) in the body. Therefore, vitamin C may help in reducing the risk of heart diseases. However, they may act as pro-oxidants which stimulate the formation of free radicals when there is a high dose of vitamin C. Until the optimum intake of vitamin C is being determined, ability of vitamin C to reduce the risk of cancer is remained unclear (Whitney et al., 2011). 2.1.3 α-Tocopherol (Vitamin E) Diagram 2.3: Structure of α-tocopherol, vitamin E As an antioxidant, vitamin E is a vital compound involved in the termination of free radicals before the mechanism of free radicals is started. This is due to the self-conversion ability of vitamin E into a free radical species and thus, termination mechanism occurs when these two free radicals react to become a molecule which is not a free radical. So, the chain reactions will be terminated. Some researches show that vitamin E may help in the prevention of heart diseases by protecting LDL from oxidation and reducing the inflammation (Groff et al, 2009). This is because oxidation of LDL may leads to atherosclerosis. Several clinical studies completed over the past 5 years, however, have not shown any beneficial effects of vitamin E in decreasing risk of death from heart diseases. Therefore, the role of vitamin E in reducing the risk of heart disease still remains uncertain. However, some studies proved that vitamin E exerts an important antioxidant effect in the lungs, the organ with the highest concentration of oxygen (Kohlmeier, 2009). Vitamin E also protects the lungs from strong oxidants contributed by the air pollutants and as a result, the risk of lung cancer and bronchitis is also believed to be decreased (Whitney et al, 2013). 2.1.4 Role of Natural Antioxidants in Food -carotene is always being used as the colouring chemicals in the food industry. This is because -carotene gives a bright orange colour for the food, mostly in beverage production. The pigmentation given by the -carotene also give a great pH and heat stability. Besides, it also acts as pro-vitamin A which is used for fortification of multivitamin juices and preparation of health supplement food. For ascorbic acid, it is used widely in the food not only because of their high nutrient value but also because of their functional contributions to different product quality. Addition of ascorbic acid is very common in the manufacture of beverages, especially for those fruit juices. Ascorbic acid can inhibit the enzymatic browning reaction which occurs naturally in the raw fruit tissues. Browning reaction will lead to formation of melanin and cause the brown pigmentation in the manufacture of fruit juices. Thus, by adding ascorbic acids, the nutritional values can be restored and it also contributes to the products’ appearances. Ascorbic acid is also widely used in the raw meat industry due to its antioxidant properties. It can be added into the raw meat during storage to prevent oxidation and colour fading which cause the meat to become brown in colour. However, for cured meat, ascorbic acid is added to accelerate the oxidation of the meat tissue. This is because the oxidation imparts the meats to their characteristic colour. Lastly, tocopherol is always used as antioxidants for protecting the oxidation-sensitive fatty products. This ensures the better quality of the oil or fatty products and also prolongs their shelf life at the same time. In addition, it also can be used for fortification of some food products such as children’s food, packaged cereals and milk powder. Furthermore, vitamin E also can be added in the vegetable oil to keep them from being oxidized and turn rancid. Likewise, it protects vitamin A in foods from being oxidized. This makes vitamin E a useful food preservative. 2.2 Synthetic Antioxidants Synthetic antioxidants are derived from phenolic structures. Having a phenolic configuration inside its molecular structure is also form synthetic antioxidant. (Sanhueza et al, 2000). There are many types of synthetic antioxidant, however the most common as synthetic antioxidants are tertiary-butyl-4-hydroxyanisole or butylated hydroxyanisol (BHA) and 2,6di-tertiary-butyl-4-methylphenol or butylated hydroxytoluene (BHT). 2.2.1 Benefits and Detriments of Synthetic Antioxidants Diagram 2.4: Structure of butylated hydroxyanisol (BHA) Diagram 2.5: Structure of butylated hydroxytoluene (BHT) BHA and BHT are commonly used in food certainly because of their beneficial effects. However, they have an optimum dose to consume or it will cause negative effects if consumption exceeds certain dosage. Both BHA and BHT can be added in food up to 0.02 %. BHA and BHT have good solubility in oils and fats. This allows them to be absorbed easily by body and stored in the liver. A small portion of BHA and BHT can reduce risks of majority types of cancer. Due to their good solubility in oils and fats, they can retard the oxidation of vitamin A, fats and vegetable oil. However, they can cause a fatty liver and hypercholesterolemia (presence of high level of cholesterol in blood) and this slows down the ability of liver to metabolize alcohol. BHA and BHT have been known to impair blood clotting when consumed in high quantities, and promote tumour growth. (Renouard, 2013) 2.2.2 Roles of Synthetic Antioxidants in Food Both BHA and BHT are used in baked food because of their thermal stability. BHA can also be added in packaging food to provide protection inside the package through volatilization, while BHT is usually mix with BHA to create greater antioxidant activity. BHA and BHT can be added to frozen foods, beers, cereals, fried foods, instant noodles and canned food. In frozen foods, BHA and BHT are effective antioxidant for natural animal fats and FDA-approved for inclusion in meat and meat products. Though fresh cut meats are unlikely to contain neither BHA nor BHT, processed meat products, like sausage or packaged frozen meat-based meals, like chicken nuggets or pre-cooked chicken wings, contain BHA and BHT in order to stabilize the natural animal fats and any added fats. Meat products that are prebaked or fried contain higher levels of BHA and BHT due to the additional fat and oil used during processing. (Wahlig, 2011) As in beers, both BHA and BHT stabilize yeast as effectively as they stabilize fat. Alcoholic beverages that use yeast as a fermentation agent use BHA/BHT to slow down the aging process of the food so that the beverage maintains its intended taste and alcohol content for an extended period of time. BHA/BHT is approved for inclusion in beer to inhibit the yeast action of the beverage. (Wahlig, 2011) 3. Conclusion From the elucidation above, it can be concluded that antioxidant can be divided into two types which are natural and synthetic antioxidant. They can be found in different kinds of food. Natural antioxidant plays its role in fruits and vegetables whereas synthetic antioxidants are applied in frozen foods, meats, and beers. Both types of antioxidant give a huge contribution in food that humans consume especially in terms of preventing diseases such as cancer and heart disease. Nevertheless, the consumption of antioxidant in diets must be at an optimum level. This is because the excess intake of antioxidant particularly synthetic antioxidant may bring various side effects to human body which can further lead to serious illness. The mechanism, benefits, detriments, and both types of antioxidant role in food have been explained above. Thus, the objective of this essay is achieved. 4. References Chen, J.F., 2004. Enjoying Health & Longevity with Nutritional Immunology, pg 99-101, 172-176. Gropper, S.S., Smith, J.L., Groff, J.L., 2009. Advanced Nutrition and Human Metabolism, 5th Edition, pg 386-388, 404-406, 421, 425. Kohlmeier, M., 2009. Nutrient Metabolism, pg 457-464,549. Mckinney, C. 2013. The Positive Effects of BHT and BHA [eHow] [online] Available at: http://www.ehow.com/info_8443550_positive-effects-bht-bha.html [Accessed: 7 Oct 2013]. Nagaoka, S., Masaki, H., Aoyama, Y. and Yoshida, A. n.p. Effects of Excess Dietary Tyrosine or Certain Xenobiotics on the Cholesterogenesis in Rats. [e-book] Japan: p. 726. [Accessed: 8 Oct 2013]. Organicconsumers.org. 2013. Synthetic Antioxidants Can Harm Your Health. [online] Available at: http://www.organicconsumers.org/articles/article_4331.cfm [Accessed: 3 Oct 2013]. Wahlig, H. 2011. Foods That Have BHA. [online] Available at: http://www.livestrong.com/article/518584-foods-that-have-bha/#ixzz2hCbey5js [Accessed: 9 Oct 2013]. Race, S. 2009. The truth about BHA, BHT, TBHQ and other antioxidants used as food additives. [e-book] Tigmor Books. Available at: http://www.foodcanmakeyouill.co.uk/library/content/Antioxidants.pdf [Accessed: 3 Oct 2013]. Renouard, C. 2013. 8 readily available foods in the US that are banned in other countries Deseret News. [online] Available at: http://www.deseretnews.com/top/1603/6/BHAand-BHT-8-readily-available-foods-in-the-US-that-are-banned-in-other-countries.html [Accessed: 9 Oct 2013]. Sanhueza, J., Nieto, S. and Valenzuela, A. 2000. Thermal stability of some commercial synthetic antioxidants. Journal of the American Oil Chemists' Society, 77 (9), pp. 933936. Available from: doi: 10.1007/s11746-000-0147-9 [Accessed: 3 Oct 2013]. Whitney, E., Rolfes, S.R., 2013. Understanding Nutrition, 13th Edition, International Edition, pg 360-363. Whitney, E., DeBruyne, L.K., Pinna, K., Rolfes, S.R., 2011. Nutrition for Health and Health Care, 4th Edition, pg 204.210,221,268.