Priniciple Investigator`s Biosafety Manual Template
advertisement

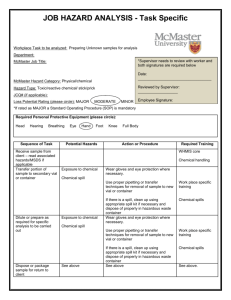
Biosafety Manual LLaabb LLooccaattiioonn__________________________________________________________________________________ P Prriinncciippllee IInnvveessttiiggaattoorr________________________________________________________________________________ E Em meerrggeennccyy P Phhoonnee##________________________________________________________________________________ LLaabb M Maannaaggeerr________________________________________________________________________________________ E Em meerrggeennccyy P Phhoonnee##____________________________________________________________________________________ D Daattee ooff R Reevviissiioonn ______________________________ IIB BC CA Apppprroovvaall##__________________________________ This Biosafety Manual Template has been developed following the principles of biosafety given in CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories (BMBL) – 5th Edition and NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines). This manual is designed to assist Principle Investigators by allowing designated sections to be modified, making the manual lab specific. This will then provide a more useful training tool and reference for the laboratory staffs. Special thanks go to the University of Maryland for supplying a format and information for this template. TABLE OF CONTENTS: 1. Important Telephone Numbers 2. Laboratory Biosafety Level Designation 3. Lab Staff Training 4. Emergency Procedures 5. Lab Specific Spill Clean-up Procedures 6. Lab specific Decontamination and Disposal Procedures 7. Responsibilities 8. Biosafety Containment Levels- Additional Reference 9. Useful Web sites PI to provide specific information to sections: 1,2,3,5 & 6 1. IMPORTANT TELEPHONE NUMBERS IN THE EVENT OF AN EMERGENCY (POLICE-FIRE-MEDICAL) DIAL 911 EAST CAMPUS DIAL 9-911 Please Note: If using a cell phone on the Uptown or Downtown Campuses, dial 442-3131, for University Police Department. ENVIRONMENTAL HEALTH AND SAFETY OFFICE – 442-3495 POWER PLANT (24/7) – 442-3444 EAST CAMPUS BOILER HOUSE (24/7) – 257-2036 SHOP COORDINATOR’S OFFICE – 442-3480 ARAMARK – for non-emergency repairs 591-8400 (East Campus only) Principle Investigator – Lab staff Name 1. 2. 3. 4. 5. - Phone number 2. LLaabboorraattoorryy B BLL D Deessiiggnnaattiioonn This laboratory is Biosafety Level (BL) PI Check one _______ Biosafety Level 1 (BL-1) is the basic level of protection and is appropriate for agents that are not known to cause disease in normal, healthy humans. ______ Biosafety Level 2 (BL-2) is appropriate for handling moderate-risk agents that cause human disease of varying severity by ingestion or through percutaneous or mucous membrane exposure. ________Biosafety Level 2+ (BL2+) is BL2 with stipulations. Agents used can cause serious disease, therefore control of the hazard is achieved by strictly following BL3 work practices and procedures in a BL2 facility. __N/A___ Biosafety level 3 (BL-3) is appropriate for agents with a known potential for aerosol transmission, for agents that may cause serious and potentially lethal infections and that are indigenous or exotic in origin. __N/A___ Biosafety Level 4 (BL-4) Exotic agents that pose a high individual risk of life-threatening disease by infectious aerosols and for which no treatment is available are restricted to high containment laboratories that meet this standard. BIOSAFETY LEVEL 3 & 4 WORK IS NOT CURRENTLY PERMITTED AT THE UNIVERSITY AT ALBANY FROM CDC/NIH BMBL – 5th Edition - Summary of BSL’s Criteria 3. Lab Staff Training Lab Specific: ____________Agent specific- handling/exposure concerns/health monitoring (if warranted) ____________Chemical handling- handling/exposure concerns (MSDS) ____________Emergency Procedures ____________Spill Procedures ____________Decontamination Procedures ____________Disposal Protocols University/Regulatory: ____________Lab safety ____________Bloodborne Pathogens (if warranted) ____________Radiation Safety (if warranted) ____________Fire Extinguisher The PI is responsible for the successful completion of the required training for all laboratory staff, as checked above. Training is available on-line through or in real time by EH&S. Please contact EH&S for assistance. 4. Emergency Procedures IN THE EVENT OF A FIRE: Pull alarm box at nearest exit. Close all fire doors behind you. Evacuate building. Do not use the elevators. Meet outside the building at a designated location. Call 911 (Uptown or Downtown Campus)or 9-911 (East Campus) IN THE EVENT OF A SPILL OF BIOHAZARDOUS MATERIALS: Wear gloves and a lab coat. Use forceps to pick up broken glass and discard into a Sharps container. Cover spilled material with paper towels. Add diluted disinfectant in sufficient quantity and allow sufficient time to ensure effective microbial inactivation. Dispose of towels in biohazard waste container. Wipe spill area with diluted disinfectant. Wash hands with soap and water when finished. 5. SPECIFIC SPILL CLEANUP GUIDELINES Lab specific procedure(s) in the event of a spill: (PI to detail) General guidelines for spill clean-up: Spill of BSL-1 Material 1. Wearing gloves and a lab coat, pick up broken glass with forceps and place in a Sharps container. 2. Absorb the spill with paper towels or other absorbent material. 3. Discard these contaminated materials into biohazard waste container. 4. Wipe the spill area with the appropriate dilution of a disinfectant effective against the organism. 5. Autoclave all materials worn or used to clean up the spill. 6. Wash hands with soap and water. Spill of Human Blood 1. Wear disposable gloves and lab coat to clean up spill. If there is a chance of a splash of blood (e.g. puddle or pool of blood), safety glasses, goggles, or a face shield should be worn. 2. If broken glass is present, use forceps to pick up and place in sharp’s container. 3. Absorb blood with paper towels and discard in biohazard/medical waste container. 4. Using a detergent solution, clean the spill site of visible blood. 5. Wipe the spill site with paper towels soaked in a disinfectant such as a 10% solution of freshly made bleach solution. 6. Discard all contaminated materials into a biohazard/medical waste container. 7. Wash hands with soap and water. Blood spill cleanup procedures are also laid out in detail in the University at Albany’s Bloodborne Pathogens Policy available at http://www.albany.edu/ehs/occ_bloodpathogens.html Spill of BSL-2 Material 1. Keep other workers out of the area to prevent spreading the spilled material. Post warning sign, if needed. 2. If clothing is contaminated, remove and put into a biohazard bag for decontamination later. 3. Wash hands and exposed skin and immediately inform the P.I. of the spill. Exposure plan should be implemented. 4. The person cleaning up the spill should put on protective clothing (lab coat, gloves and if needed, face/eye protection and shoe covers) and assemble clean-up materials (disinfectant, autoclavable container or bag, forceps, sharps container and paper towels). 5. Pick up any broken glass with forceps and dispose in Sharps container. 6. Cover the spill with paper towels and the appropriately diluted disinfectant. 7. After at least 20 minutes contact time, pick up paper towels and re-wipe the spill area with diluted disinfectant. 8. Collect all contaminated materials into a biohazard/medical waste container and autoclave. 9. Wash hands with soap and water. Spill in a Biological Safety Cabinet 1. Leave the cabinet fan running. 2. Wearing gloves and lab coat spray or wipe cabinet walls, work surfaces and equipment with the appropriate disinfectant. If necessary, flood work surfaces, as well as drain pans and catch basins below the work surface with disinfectant. Allow at least 20 minutes contact time. 3. Soak up the disinfectant and spill with paper towels, and drain catch basin into a container. Lift front exhaust grille and tray, and wipe all surfaces. Ensure that no paper towels or solid debris are blown into the area below the grille. 4. Surface disinfect all items that may have been spattered before removing them from the cabinet. 5. Discard all clan up materials into a biohazard waste container. Wash hands and exposed skin areas with soap and water. 6. If the spill overflows into the interior of the cabinet, it may be necessary to do a more extensive decontamination of the cabinet. 6. Decontamination and Disposal Lab specific procedure(s) for decontamination and Disposal: (PI to detail) General Guidelines for decontamination and Disposal Sterilization, disinfection, and antisepsis are all forms of decontamination. Sterilization implies the killing of all living organisms. Disinfection refers to the use of antimicrobial agents on inanimate objects; its purpose is to destroy all non-spore forming organisms. Antisepsis is the application of a liquid antimicrobial chemical to living tissue. Chemical Disinfectants Chemical disinfectants are used to render a contaminated material safe for further handling, whether it is a material to be disposed of as waste, or a laboratory bench on which a spill has occurred. It is important to choose a disinfectant that has been proven effective against the organism being used. Chemical disinfectants are registered by the EPA under the following categories: o o o Sterilizer or Sterilant - will destroy all microorganisms including bacterial and fungal spores on inanimate surfaces. Disinfectant - will destroy or irreversibly inactivate specific viruses, bacteria, and pathogenic fungi, but not bacterial spores. Hospital Disinfectant - agent shown to be effective against S. aureus, S. choleresis and P. aeruginosa. It may be effective against M. tuberculosis, pathogenic fungi or specifically named viruses. Disinfectants Commonly Used in the Laboratory Iodophors o Recommended dilution is 75 ppm, or approximately 4.5 ml/liter water. o Effective against vegetative bacteria, fungi, and viruses. o Effectiveness reduced by organic matter (but not as much as with hypochlorites). o Stable in storage if kept cool and tightly covered. o Built-in color indicator; if solution is brown or yellow, it is still active. o Relatively harmless to humans. Manufacturer's recommended dilution is 3 ounces (90 ml) into 5 gallons water, or approximately 4.5 ml/liter. For porous surfaces, use 6 ounces into 5 gallons water. Hypochlorites (bleach) o Working dilution is 1:10 to 1:100 in water. o Effective against vegetative bacteria, fungi, most viruses at 1:100 dilution. o Effective against bacterial spores at 1:10 dilution. o Very corrosive. o Rapidly inactivated by organic matter. o Solutions decompose rapidly; fresh solutions should be made daily. Alcohols (ethanol, isopropanol) o The effective dilution is 70-85%. o Effective against a broad spectrum of bacteria and many viruses. o Fast acting. o Leaves no residue. o Non-corrosive. o Not effective against bacterial spores. Important Characteristics of Disinfectants Hypochlorites “Bleach” Shelf Life >1 week Iodoform “Wescodyne” Ethyl Alcohol X X Corrosive X X Residue X X Inactivation by Organic Matter X X Skin Irritant X X Respiratory Irritant X Eye Irritant X X X Toxic X X X Dilution of Disinfectants o Chlorine Compounds (Household Bleach) Dilution in Water %Available Available Chlorine Chlorine mg/l or ppm Not diluted 5.25 50,000 1/10 0.5 5,000 1/100 0.05 500 Bleach solutions decompose at room temperature and should be made fresh daily. However, if stored in tightly closed brown bottles, bleach solutions retain activity for 30 days. The use concentration is dependent on the organic load of the material to be decontaminated. Use a 1% solution to disinfect clean surfaces, and 10% solution to disinfect surfaces contaminated with a heavy organic load. To disinfect liquid biological waste before disposal, add concentrated bleach to a final concentration of 1%. o o Iodophor o Manufacturer's recommended dilution is 3 ounces (90 ml) into 5 gallons water, or approximately 4.5 ml/liter. For porous surfaces, use 6 ounces into 5 gallons water. Alcohols o Ethyl alcohol and isopropyl alcohol diluted to 70 - 85% in water are useful for surface disinfection of materials that may be corroded by a halogen or other chemical disinfectant. Autoclaving Procedures: The autoclaves at the University at Albany are not certified to handle regulated medical waste. If a waste is by definition a “regulated medical waste”, it must still go out as regulated medical waste, even after it has been autoclaved. Autoclaves use pressurized steam to destroy microorganisms, and are the most dependable system available for the decontamination of laboratory waste and the sterilization of laboratory glassware, media, and reagents. For efficient heat transfer, steam must flush the air out of the autoclave chamber. Before using the autoclave, check the drain screen at the bottom of the chamber and clean if blocked. If the sieve is blocked with debris, a layer of air may form at the bottom of the autoclave, preventing efficient operation. Container Selection o o o o o o Polypropylene bags. Commonly called biohazard or autoclave bags, these bags are able to withstand autoclaving and are tear resistant, but can be punctured or burst in the autoclave. Therefore, place bags in a rigid container such as a polypropylene or stainless steel pan during autoclaving. Bags are available in a variety of sizes, and some are printed with an indicator that changes color when processed. These bags can be purchased from C.A.S. Stores or from vendors like Lab Safety Supply or Krackeler Scientific. Polypropylene bags are impermeable to steam, and for this reason should not be twisted and taped shut, but gathered loosely at the top and secured with a large rubber band or autoclave tape. This will create an opening through which steam can penetrate. Polypropylene containers and pans. Polypropylene is a plastic capable of withstanding autoclaving, but resistant to heat transfer. Therefore, materials contained in a polypropylene pan will take longer to autoclave than the same materials in a stainless steel pan. To decrease the time required to sterilize material in these containers, remove the lid (if applicable). turn the container on its side when possible. select a container with the lowest sides and widest diameter possible for the autoclave. o Stainless steel containers and pans. Stainless steel is an efficient conductor of heat and is less likely to increase sterilizing time, though is more expensive than polypropylene. “You must sign the Log Book each time you use the autoclave.” Preparation and Loading of Materials - Fill liquid containers only half full. o o o o o Always put bags of biological waste into pans to catch spills. Loosen caps, or use vented closures. Position biohazard bags on their sides, with the bag neck taped loosely. Leave space between items to allow steam circulation. Household dishpans melt in the autoclave. Use autoclavable polypropylene or stainless steel pans. Cycle Selection o o o Use liquid cycle (slow exhaust) when autoclaving liquids, to prevent contents from boiling over. Select fast exhaust cycle for glassware. Use fast exhaust and dry cycle for wrapped items. Time Selection o o o Take into account the size of the articles to be autoclaved. A 2-liter flask containing 1 liter of liquid takes longer to sterilize than four 500 ml flasks each containing 250 ml of liquid. Material with a high insulating capacity (animal bedding, high-sided polyethylene containers) increases the time needed for the load to reach sterilizing temperatures. Bags of biological waste should be autoclaved for 50 minutes to assure decontamination. Removing the Load o o o o o Check that the chamber pressure is zero. Wear lab coat, eye protection, heat insulating gloves, and closed-toe shoes. Stand behind door when opening it. Slowly open door only a crack. Beware of rush of steam. After the slow exhaust cycle, open autoclave door and allow liquids to cool for 20 minutes before removing. Monitoring Autoclaves used to decontaminate laboratory waste should be tested periodically to assure effectiveness. At a minimum, autoclave indicator tape should be used on all items being autoclaved. However, there are two other types of tests that can be used:1) a chemical indicator that fuses when the temperature reaches 121°C, and 2) heat-resistant spores (Bacillus stearothermophilis) that are killed by exposure to 121°C for approximately 15 minutes. Both types of tests should be placed well down in the center of the bag or container of waste, at the point slowest to heat. The chemical test should be used first to determine that the temperature in the center of the container reaches 121°C. Ampules of heat-resistant spores should be used in subsequent test runs to determine the amount of time necessary to achieve sterilization. If you need additional assistance when autoclaving, please contact the building managers. Caution - Autoclaves May Cause Serious Burns To Prevent Injury: o o o o o o o o Loosen screw caps on bottles and tubes of liquids before autoclaving. Check that chamber pressure has returned to zero before opening door. Wear eye and face protection. Stand behind door when opening it. Slowly open door only a crack. Beware rush of steam. Keep face away from door as it opens. Escaping steam may burn face. Wait 5 minutes after opening door before removing liquids. Liquids removed too soon may boil up and out of container, burning operator. Use and Disposal of Sharps: The purchase, possession, use, destruction and disposal of any and all instruments and/or supplies, which could be used as a means of injecting narcotic drugs are governed by the “University at Albany Guidelines and “Approved User” Application for the Procurement, Storage, Use, Destruction and Disposal of Hypodermic Supplies”. These guidelines are overseen by the Director of C.A.S. Technical Services and his designees. Please contact the Director, if you have any questions pertaining to the use of hypodermic materials. To prevent needle stick injuries: o o o Avoid using needles whenever possible. Do not bend, break, or otherwise manipulate needles by hand. Do not recap needles by hand. Do not remove needles from syringes by hand. o Immediately after use, discard needle and syringe (whether contaminated or not) into puncture resistant sharps containers. Never discard sharps into regular trash. Never discard sharps into bags of biological waste. Use care and caution when cleaning up after procedures that require the use of syringes and needles. o o o o o Do not overfill the sharps containers. Close completely when they are 3/4 full Locate sharps containers in areas in which needles are commonly used. Make containers easily accessible. Sharps containers may be purchased from C.A.S. Stores or from vendors such as Lab Safety Supply or Fisher Scientific. In the event of a needle stick injury: Wash thoroughly with soap and water. Notify supervisor and go immediately to University Health Center (UHC). If UHC is closed, go to the most convenient local emergency room. Biological Waste Disposal Procedures o o o o All biological waste from BSL1 and BSL2 laboratories must be decontaminated prior to disposal. Decontamination and disposal are the responsibility of the person/laboratory generating the waste. Collect disposable, solid materials contaminated by an infectious agent, excluding sharps, or broken or unbroken glass, into an autoclave bag within a sturdy container. When full, these bags are autoclaved, cooled, and then placed in the building's dumpster. Decontaminated liquids containing a biological agent by the addition of a chemical disinfectant such as sodium hypochlorite (household bleach) or an iodophor, or by autoclaving, then dispose of by pouring down the sink. It is not necessary to autoclave liquids that have been chemically disinfected. However, if bleach has been used in the tray used to collect lab ware that will later be autoclaved, sodium thiosulfate must be added to the bleach to prevent the release of chlorine gas during autoclaving. Reusable Lab ware Items such as culture flasks and centrifuge bottles are decontaminated by lab personnel before washing by one of two methods. o o Autoclave items that have been collected in autoclavable container. Chemically disinfect items by soaking in diluted disinfectant for one hour before washing. Disposal of Blood Products and Body Fluids o o o All human blood and other potentially infectious materials should be handled using Standard Precautions. These materials must be disposed of as Regulated Medical Waste. Please see Building Manager for Regulated Medical Waste Disposal. Discard disposable items contaminated with human blood or body fluids (excluding sharps and glassware) into the incinerator boxes that are available from CAS. Do not overfill boxes or use without the plastic liners provided with them. These boxes may be used for temporary storage and accumulation of waste. When full, close and seal the plastic liner and box. Disposal of Sharps and Disposable Glassware o o o Discard all needles, needle and syringe units, scalpels, and razor blades, whether contaminated or not, directly into rigid, red, labeled sharps containers. Do not recap, bend, remove or clip needles. Sharps containers should not be overfilled. Alternatively, closed sharps containers may be packaged in incinerator boxes (Section III above). Sharps containers may be purchased from CAS Stores. Uncontaminated pasteur pipettes and broken or unbroken glassware are discarded into containers specifically designed for broken glass disposal, or into heavy-duty cardboard boxes that are closeable. When boxes are full, tape closed and place in the building's dumpster. Contaminated pasteur pipettes, and broken or unbroken glassware may be treated in one of two ways: o Discarded into approved sharps containers, as in Section A above, or o Decontaminated by autoclaving or chemical disinfection, and then discarded into glass disposal boxes as in Section B above. o Sharps that are contaminated with radioactive materials or hazardous chemicals should be discarded into separate sharps containers labeled with the name of the isotope or chemical. Contact EHS at 442-3495 for disposal information. Multi-hazard or Mixed Waste o o o o Avoid generating mixed waste if possible. Keep volume to minimum. Do not autoclave mixed waste. When discarding waste containing an infectious agent and radioactive material, inactivate the infectious agent first, and then dispose of as radioactive waste. Seek advice from the RSO at 442-3495 before beginning inactivation procedures. When discarding waste containing an infectious agent and a hazardous chemical, inactivate the infectious agent first, and then dispose of as chemical waste. Seek advice before beginning inactivation procedures. Contact EHS at 442-3495 for instructions. VI. Disposal of Animal Tissues, Carcasses and Bedding o o o Disposal of animal carcasses/tissues is coordinated through the ACF, in which the animal is housed. Place animal carcasses/tissues into plastic bag. Double-bag when carcass contains zoonotic agent (transmissible from animals to humans). Place bag in freezer. Disposal Containers Each laboratory is responsible for purchasing containers for the disposal of biological waste, Except incinerator boxes (with liners), which will be provided by The following types of containers are available: o Sharps containers may be purchased from local sources (including CAS Stores) as well as from laboratory product distributors. They are available in various sizes, and o o o should be puncture resistant, red, labeled as "Sharps," and have a tightly closing lid. Do not purchase "needle-cutter" devices, which may produce aerosols when used. Biohazard Autoclave Bags may be purchased from various laboratory product distributors, such as Fisher Scientific, VWR, and Baxter. Be sure to select polypropylene bags which are able to withstand autoclaving. They should be placed inside a rigid container with lid while waste is being collected. Incinerator Boxes are provided by. A plastic liner (also provided by ) must be used to prevent contamination of the box. Glass Disposal Boxes may be purchased from Chemistry Stores and various laboratory product distributors. Alternatively, heavy-duty, closeable cardboard boxes may be used for disposal of broken glass. For What to do with Filled Waste Containers, review The University’s Chemical Hygiene Plan at: http://www.albany.edu/ehs/chemicalhygiene07.pdf 7. RESPONSIBILITIES The Principal Investigator (PI) is directly and primarily responsible for the safe operation of the laboratory. His/her knowledge and judgment are critical in assessing risks and appropriately applying the recommendations in this manual. However, safety is a shared responsibility among all of the laboratory staff. There are several resources for the PI to use when addressing these responsibilities. There is the Institutional Biosafety Committee (IBC), the Biosafety Officer and the Environmental Health and Safety Office (EH&S). Specifically, PIs shall: o o o o o o Address the risks of their experiments and where necessary, determine the appropriate biosafety level for their research; Develop and implement safety protocols specific to their research and add them to the Biosafety Manual Template; Ensure the safe operation of their laboratory; Train laboratory personnel in safe work practices, also in safe work practices specific to the laboratory’s research, this training should be documented in writing; Comply with all applicable state and federal regulations, guidelines and recommendations. Submit to the IBC the appropriate IBC Forms for proposed research involving biohazardous materials before the research has begun and to wait for IBC approval before beginning the proposed research. The IBC shall: o o o o o o Review rDNA research conducted at or sponsored by the University at Albany for compliance with NIH Guidelines, and approve those research projects that conform with the NIH Guidelines; Review research involving potentially infectious agents conducted or sponsored by the University at Albany for compliance with the guidelines in Biosafety in Microbiological and Biomedical Laboratories (BMBL), and approve those research projects that are found to conform with the recommendations in BMBL; Notify the PI of the results of the IBC’s review and approval; Where necessary or appropriate, change the biosafety level of the research; Report any significant problems or violations of the NIH Guidelines and any significant research-related accidents or illness to the appropriate Institutional official and to the NIH Office of Biotechnology Activities (OBA) within 30 days; and Follow the guidelines for membership defined by NIH, with the additional requirement of one representative for the University at Albany’s IACUC. The EH&S Office, through the Biosafety Officer, shall: o o o o o o Prepare a Template Biosafety Manual, with revisions as necessary, to be used by the PIs as a Template; Ensure that all PIs using biohazardous materials and all IBC members have access to the Biosafety Manual Template; Investigate accidents involving potentially infectious agents; Provide or coordinate biosafety and bloodborne pathogens training as requested; Assist PIs with risk assessment; and Provide access to medical surveillance and vaccinations, as required by the OSHA Bloodborne Pathogens Standard (CFR 1910.1030) and as recommended in the BMBL and NIH Guidelines. Laboratory Personnel shall: o o Follow the safety procedures and protocols found in the Biosafety Manual Template; and Follow the specific procedures and protocols developed by their PI for the research conducted in their laboratory, and sign off on any training received from their PI. 8. Biosafety Level Reference DEFINITIONS AND PRINCIPLES OF BIOSAFETY What is biosafety? According to CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories (BMBL), it is “the discipline addressing the safe handling and containment of infectious microorganisms and hazardous biological materials.” The principles of biosafety are containment and risk assessment. From the 5th Edition of the BMBL, “the fundamentals of containment include the microbiological practices, safety equipment, and facility safeguards that protect laboratory workers, the environment, and the public from exposure to infectious microorganisms that are handled and stored in the laboratory. Risk assessment is the process that enables the appropriate selection of microbiological practices, safety equipment, and facility safeguards that can prevent laboratory-associated infections (LAI).” “The primary risk criteria used to define the four ascending levels of containment, referred to as biosafety levels 1 through 4, are infectivity, severity of disease, transmissibility, and the nature of the work being conducted. Another important risk factor for agents that cause moderate to severe disease is the origin of the agent, whether indigenous or exotic. Each level of containment describes the microbiological practices, safety equipment and facility safeguards for the corresponding level of risk associated with handling a particular agent. The basic practices and equipment are appropriate for protocols common to most research and clinical laboratories. The facility’s safeguards help protect non-laboratory occupants of the building and the public health and environment. Biosafety level 1 (BSL-1) is the basic level of protection and is appropriate for agents that are not known to cause disease in normal, healthy humans. Biosafety level 2 (BSL-2) is appropriate for handling moderate-risk agents that cause human disease of varying severity by ingestion or through percutaneous or mucous membrane exposure. Biosafety level 3 (BSL-3) is appropriate for agents with a known potential for aerosol transmission, for agents that may cause serious and potentially lethal infections and that are indigenous or exotic in origin. Exotic agents that pose a high individual risk of life-threatening disease by infectious aerosols and for which no treatment is available are restricted to high containment laboratories that meet biosafety level 4 (BSL-4) standards.” BMBL – 5th Edition BIOSAFETY CONTAINMENT LEVELS Four levels of biosafety are defined in the BMBL. The levels, designated in ascending order by degree of protection provided to personnel, the environment, and the community, are combinations of laboratory practices, safety equipment, and laboratory facilities. Microbiological work done at the University at Albany is conducted at BSL-1 or BSL-2 containment. There are no BSL-3 or BSL-4 laboratories at the University at Albany. Biosafety Level -1 BSL-1 is appropriate for laboratories in which work is done with well-characterized agents not known to cause disease in healthy adult humans. BSL-1 represents a basic level of containment that relies on standard microbiological practices with no special primary or secondary barriers recommended, other than a sink for handwashing. The laboratory is not necessarily separated from the general traffic patterns in the building. The following Standard Microbiological Practices apply to all biosafety levels. There are additional practices recommended for the ascending biosafety levels. STANDARD MICROBIOLOGICAL PRACTICES: o o o o o o o o o Access to the laboratory is limited or restricted at the discretion of the P.I. when experiments or work with cultures and specimens are in progress. Persons must wash their hands after they handle viable materials and animals, after removing gloves, and before leaving the laboratory. Eating, drinking, smoking, handling contact lenses, and applying cosmetics and storing food for human consumption are not permitted in the laboratory. Mouth pipetting is prohibited; mechanical pipetting devices must be used. Policies for the safe handling of sharps must be developed and implemented. All procedures are performed carefully to minimize the creation of splashes and/or aerosols. All work surfaces are to be decontaminated after completion of work and after any spill or splash of potentially infectious material with appropriate disinfectant. All cultures, stocks, and other regulated wastes are decontaminated before disposal by an approved decontamination method, such as autoclaving. Materials to be decontaminated outside of the immediate laboratory are to be placed in durable, leakproof container and closed for transport from the laboratory. Materials to be decontaminated off-site must be packaged in accordance with applicable state and federal regulations before removal from the facility. A sign incorporating the universal biohazard symbol must be posted at the entrance to the laboratory when infectious agents are present. o The sign may include the name of the agent(s) in use, and should include the name and phone number of the P.I. or other responsible personnel. o An effective integrated pest management program is required. o The P.I. must ensure that his/her laboratory personnel receive appropriate training regarding their duties, the necessary precautions to prevent exposures, and exposure evaluation procedures. This training should be documented in writing. Personnel must receive annual updates or additional training when procedural or policy changes occur. Personal health status may impact an individual’s susceptibility to infection, ability to receive immunizations or prophylactic interventions. Therefore, all laboratory personnel and particularly women of child-bearing age should be provided with information regarding immune competence and conditions that may predispose them to infection. Individuals having these conditions should be encouraged to self-identify. SPECIAL PRACTICES: o None required. SAFETY EQUIPMENT (PRIMARY BARRIERS AND PERSONAL PROTECTIVE EQUIPMENT): o o o o Special containment devices or equipment, such as biological safety cabinets (BSCs) are not generally required for working in a BSL-1 laboratory. Laboratory coats, gowns or uniforms are recommended to prevent contamination of personal clothing. Gowns that secure in the back with gathered cuffs are recommended. Disposable coats, gowns or uniforms are recommended. These should not be worn outside of the laboratory. Protective eyewear, such as chemical splash goggles, must be worn by laboratory personnel conducting procedures that have the potential to create splashes of microorganisms or other hazardous materials. Personnel wearing contact lenses in the laboratory must also wear eye protection. Gloves must be worn to protect hands from exposure to hazardous materials and microorganisms. Glove selection should be based on an appropriate risk assessment. Long (11 – 12”), powder-free, nitrile surgical gloves are recommended. Hands must always be washed prior to leaving the laboratory. LABORATORY FACILITIES: o o o o o Laboratories must have doors for access control. Laboratories must have a sink for hand washing. The laboratory should be easily cleaned. Carpets and rugs are not appropriate for a laboratory. Chairs used in laboratory work must be covered with a non-porous material that can be easily cleaned and decontaminated with appropriate disinfectant. Laboratory furniture must be capable of supporting anticipated loads and uses. Spaces between benches, cabinets, and equipment should be accessible for cleaning. Any laboratory window that opens to the exterior should be fitted with screens. BIOSAFETY LEVEL -2 BSL-2 builds upon BSL-1. BSL-2 is suitable for work involving agents that pose moderate hazards to personnel and the environment. It differs from BSL-1 in that (1) laboratory personnel have specific training in handling pathogenic agents and are supervised by competent scientists; (2) access to the laboratory is limited when work is being conducted; and (3) all procedures in which infectious aerosols or splashes may be created are conducted in BSCs or other physical containment equipment. The following standard and special practices, safety equipment, and facility requirements apply to a BSL-2: STANDARD MICROBIOLOGICAL PRACTICES: o o All the Standard Microbiological Practices listed for a BSL-1 plus; The sign incorporating the universal biohazard sign must be posted at the entrance to the laboratory when infectious agents are present and the posted information must include: the laboratory’s biosafety level, the P.I.’s name, telephone number, and required procedures for entering and exiting the laboratory. SPECIAL PRACTICES: o o o o o o o o o All persons entering the laboratory must be advised of the potential hazards and meet specific entry/exit requirements. Laboratory personnel must be provided medical surveillance and offered appropriate immunizations for agents handled or potentially present in the laboratory. If necessary, policies and procedures must be established describing the collection and storage of serum from at-risk personnel. A laboratory-specific biosafety manual must be prepared and adopted as policy. The biosafety manual must be available and accessible. This template may be used along with the addition of specific procedures and protocols for safe handling, usage, storage and disposal of specific infectious agents used in that laboratory’s research. The P.I. must ensure that laboratory personnel demonstrate proficiency in standard and special microbiological practices before working with BSL-2 agents. Potentially infectious materials must be placed in a durable, leak proof container during collection, handling processing, storage or transport within a facility. It is recommended that the container have the universal biohazard symbol on it. Laboratory equipment should be routinely decontaminated, as well as, after spills, splashes, or other potential contamination. The equipment must also be decontaminated before repair, maintenance or removal from the laboratory. In the event of a spill of infectious material, only trained personnel may participate in the containment, decontamination and clean up of the spill. Animals and plants not associated with the work being performed in the laboratory are not permitted in the laboratory. All procedures involving the manipulation of infectious materials that may generate an aerosol should be conducted within a BSC or other physical containment devices. SAFETY EQUIPMENT (Primary Barriers and Personal Protective Equipment) o o o Whenever procedures with a potential for creating infectious aerosols or splashes are conducted or high concentrations or large volumes of infectious agents are used, a properly maintained BSC (preferably Class II), other appropriate personal protective equipment, or other physical containment devices must be used. The BSC must be certified annually and whenever it is moved. Protective laboratory coats, gowns, smocks, or uniforms designated for laboratory use must be worn while working with hazardous materials. Protective clothing must be removed when leaving the laboratory. Dispose of protective clothing appropriately, or deposit it for laundering by the institution. It is recommended that laboratory clothing not be taken home. Eye and face protection (goggles, mask, face shield or other splatter guard) must be used whenever there is a potential for splashes or sprays of infectious or other o hazardous materials when the microorganisms must be handled outside the BSC or containment device. Persons wearing contact lenses in the laboratories must also wear eye protection. ALL PPE must be disposed of with other contaminated laboratory waste or decontaminated before reuse. Gloves must be worn to protect hands from exposure to hazardous materials and glove selection should be based on appropriate risk assessment. Alternatives to latex gloves should be available. Gloves should be changed when contaminated, integrity has been compromised, or whenever necessary. If appropriate, double glove. Do not wash or reuse disposable gloves. Dispose of used gloves with other contaminated laboratory waste. ALWAYS remove gloves and wash hands after work has been completed and before leaving the laboratory. LABORATORY FACILITIES (Secondary Barriers) o o o o o o o o o o Laboratory doors should be self-closing and have locks. A sink for hand washing must be available in the laboratory. It should be located near the exit door. The laboratory should be easily cleaned and decontaminated. Carpets and rugs are not permitted. Chairs used in laboratory work must be covered with a non-porous material that can be easily cleaned and decontaminated. Spaces between benches, cabinets, and equipment should be accessible for cleaning. Laboratory furniture must be capable of supporting anticipated loads. And uses. Bench tops must be impervious to water and resistant to heat, organic solvents, acids, alkalis, and other chemicals. Laboratory windows that open to the exterior must be fitted with fly screens. BSCs must be installed so that fluctuations of the room supply and exhaust do not interfere with proper operations. BSCs should be located away from doors, windows that can be opened, heavily traveled laboratory areas, and other possible airflow disruptions. Vacuum lines should be protected with High Efficiency Particulate Air (HEPA) filters, or their equivalent. Filters must be replaced as needed. Liquid disinfectant traps may be required. An eyewash station must be readily available. HEPA filtered exhaust air from a Class II BSC can be safely re-circulated back into the laboratory environment, if the cabinet is tested and certified at least annually and operated according to manufacturer’s recommendations. A method for decontaminating all laboratory wastes should be available at the facility (e.g., autoclave, chemical disinfection, incineration or other validated decontamination method). 9. USEFUL WEB SITES University at Albany Office of Regulatory Research Compliance: http://www.albany.edu/research/compliance/ NIH Guidelines: http://www4.od.nih.gov/oba/rac/guidelines/guidelines.html BMBL: http://www.cdc.gov/od/ohs/biosfty/bmbl5/bmbl5toc.htm NIH Office of Biotechnology Activities: http://www4.od.nih.gov/oba/ CDC Select Agents Program: http://www.cdc.gov/od/sap/index.htm USDA/APHIS Select Agents Program: http://www.aphis.usda.gov/vs/ncie/bta.html CDC Permit to Import or Transport Etiologic Agents: http://www.cdc.gov/od/ohs/biosfty/imprtper.htm USDA/APHIS Permit to Import or Transport Livestock Pathogens: http://www.aphis.usda.gov/animal_health/permits/ USDA/APHIS Permit to Field Test, Import, or Transport Genetically Modified Organisms: http://www.aphis.usda.gov/brs/regulatory_activities.html Selection, Installation, and Use of Biological Safety Cabinets: http://www.cdc.gov/od/ohs/biosfty/bsc/bsc.htm