Chapter 16 - Acid-Base Equilibria and Solubility Equilibria
advertisement
AP Chemistry
Mr. Markic
Page 1 of 12
Chapter 16 - Acid-Base Equilibria and Solubility Equilibria
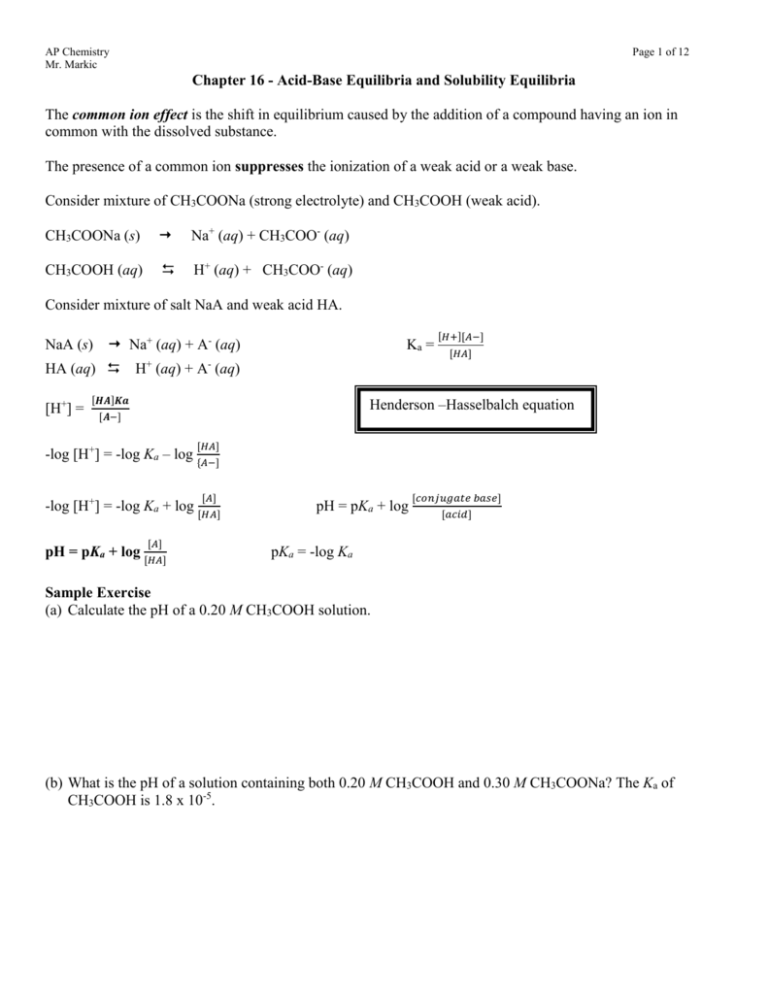
The common ion effect is the shift in equilibrium caused by the addition of a compound having an ion in
common with the dissolved substance.
The presence of a common ion suppresses the ionization of a weak acid or a weak base.
Consider mixture of CH3COONa (strong electrolyte) and CH3COOH (weak acid).
CH3COONa (s)
Na+ (aq) + CH3COO- (aq)
CH3COOH (aq)
H+ (aq) + CH3COO- (aq)
Consider mixture of salt NaA and weak acid HA.
NaA (s) Na+ (aq) + A- (aq)
Ka =
HA (aq) H+ (aq) + A- (aq)
[H+] =
[𝑯𝑨]𝑲𝒂
[𝐻+][𝐴−]
[𝐻𝐴]
Henderson –Hasselbalch equation
[𝑨−]
[𝐻𝐴]
-log [H+] = -log Ka – log {𝐴−]
[𝐴]
-log [H+] = -log Ka + log [𝐻𝐴]
[𝐴]
pH = pKa + log [𝐻𝐴]
pH = pKa + log
[𝑐𝑜𝑛𝑗𝑢𝑔𝑎𝑡𝑒 𝑏𝑎𝑠𝑒]
[𝑎𝑐𝑖𝑑]
pKa = -log Ka
Sample Exercise
(a) Calculate the pH of a 0.20 M CH3COOH solution.
(b) What is the pH of a solution containing both 0.20 M CH3COOH and 0.30 M CH3COONa? The Ka of
CH3COOH is 1.8 x 10-5.
AP Chemistry
Mr. Markic
Page 2 of 12
What is the pH of a solution containing 0.30 M HCOOH and 0.52 M HCOOK?
A buffer solution is a solution of:
1. A weak acid or a weak base and
2. The salt of the weak acid or weak base
Both must be present!
A buffer solution has the ability to resist changes in pH upon the addition of small amounts of either acid or
base.
Consider an equal molar mixture of CH3COOH and CH3COONa
Add strong acid
H+ (aq) + CH3COO- (aq)
Add strong base
OH- (aq) + CH3COOH (aq)
CH3COOH (aq)
CH3COO- (aq) + H2O (l)
Sample Exercise
Which of the following solutions can be classified as buffer systems?
(a) KH2PO4 / H3PO4
(b) NaClO4 / HClO4
(c) C5H5N / C5H5NHCl (C5H5N is pyridine, its Kb is given in Table 15.4) Explain your answer.
Which of the following are buffer systems?
(a) KF/HF
(b) KBr/HBr
(c) Na2CO3/NaHCO3
(a) Calculate the pH of a buffer system containing 1.0 M CH3COOH and 1.0 M CH3COONa.
AP Chemistry
Mr. Markic
Page 3 of 12
(b) What is the pH of the buffer system after the addition of 0.10 mole of gaseous HCl to 1.0 L of the solution?
Assume that the volume of the solution does not change when the HCl is added.
Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer system. What is the pH after the addition of 20.0 mL
of 0.050 M NaOH to 80.0 mL of the buffer solution?
HCl
H+ + Cl-
HCl + CH3COO- CH3COOH + Cl-
Review of Concepts
The following diagrams represent solution containing a weak acid HA and/or its sodium salt NaA. Which
solutions can act as a buffer? Which solution has the greatest buffer capacity? The Na+ ions and water
molecules are omitted for clarity.
Practice Exercise
Describe how you would prepare a “phosphate buffer” with a pH of about 7.40.
AP Chemistry
Mr. Markic
Page 4 of 12
Titrations
In a titration a solution of accurately known concentration is added gradually added to another solution of
unknown concentration until the chemical reaction between the two solutions is complete.
Equivalence point – the point at which the reaction is complete
Indicator – substance that changes color at (or near) the equivalence point
Slowly add base to unknown acid UNTIL the indicator changes color (pink)
Strong Acid-Strong Base Titrations
NaOH (aq) + HCl (aq) H2O (l) + NaCl (aq)
OH- (aq) + H+ (aq)
H2O (l)
Weak Acid-Strong Base Titrations
CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l)
CH3COOH (aq) + OH- (aq) CH3COO- (aq) + H2O (l)
At equivalence point (pH > 7):
CH3COO- (aq) + H2O (l) OH- (aq) + CH3COOH (aq)
Sample Exercise
Calculate the pH in the titration of 25.0 mL of 0.100 M acetic acid by sodium hydroxide after the addition to the
acid solution of
(a) 10.0 mL of 0.100 M NaOH
AP Chemistry
Mr. Markic
Page 5 of 12
(b) 25.0 mL of 0.100 M NaOH
(c) 35.0 mL of 0.100 M NaOH
Practice Exercise
Exactly 100 mL of 0.10 M nitrous acid (HNO2) are titrated with a 0.10 M NaOH solution. Calculate the pH for
(a) The initial solution
(b) The point at which 80 mL of the base has been added
(c) The equivalence point
(d) The point at which 105 mL of the base has been added
AP Chemistry
Mr. Markic
Page 6 of 12
Strong Acid-Weak Base Titrations
HCl (aq) + NH3 (aq) NH4Cl (aq)
H+ (aq) + NH3 (aq) NH4+ (aq)
At equivalence point (pH < 7):
NH4+ (aq) + H2O (l) NH3 (aq) + H+ (aq)
Calculate the pH at the equivalence point when 25.0 mL of 0.100 M NH3 is titrated by a 0.100 M HCl solution
Calculate the pH at the equivalence point in the titration of 50 mL of 0.10 M methylamine (see Table 15.4) with
a 0.20 M HCl solution.
Review of Concepts
For which of the following titrations will the pH at the equivalence point not be neutral?
(a) NaOH with HNO2
(c) KOH with HCOOH
(b) KOH with HClO4
(d) CH3NH2 with HNO3
AP Chemistry
Mr. Markic
Page 7 of 12
Acid-Base Indicators
HIn (aq)
H+ (aq) + In- (aq)
[𝐻𝐼𝑛]
[𝐼𝑛−]
[𝐻𝐼𝑛]
[𝐼𝑛−]
≥ 10 Color of acid (HIn) predominates
≤ 10 Color of conjugate base (In-) predominates
Which indicator or indicators listed in Table 16.1 would
you use for the acid-base titrations shown in:
(a) Figure 16.4?
(b) Figure 16.5?
(c) Figure 16.6?
Solubility Equilibria
AgCl (s)
Ag+ (aq) + Cl- (aq)
Ksp = [Ag+][Cl-]
Ksp is the solubility product constant
MgF2 (s) Mg2+ (aq) + 2F- (aq)
Ag2CO3 (s)
2Ag+ (aq) + CO32- (aq)
Ca3(PO4)2 (s) 3Ca2+ (aq) + 2PO43- (aq)
Ksp = [Mg2+][F-]2
Ksp = [Ag+]2[CO32-]
Ksp = [Ca2+]3[PO43-]2
AP Chemistry
Mr. Markic
Page 8 of 12
Dissolution of an ionic solid in aqueous solution:
Q < Ksp
Unsaturated solution
Q = Ksp
Saturated solution
Q > Ksp
Supersaturated solution
No precipitate
Precipitate will form
Review of Concepts
The following diagrams represent solutions of AgCl, which may also contain ions such as Na+ amd NO3- (not
shown) that do not affect the solubility of AgCl. If (a) represents a saturated solution of AgCl, classify the
other solutions as unsaturated, saturated, or supersaturated.
Molar solubility (mol/L) is the number of moles of solute dissolved in 1 L of a saturated solution.
Solubility (g/L) is the number of grams of solute dissolved in 1 L of a saturated solution.
AP Chemistry
Mr. Markic
Page 9 of 12
Sample Exercise
The solubility of calcium sulfate (CaSO4) is found to be 0.67 g/L. Calculate the value of Ksp for calcium
sulfate
The solubility of lead chromate (PbCrO4) is 4.5 x 10-5 g/L. Calculate the solubility product of this compound.
Using the data in Table 16.2, calculate the solubility of copper (II) hydroxide, Cu(OH)2 in g/L.
What is the solubility of silver chloride in g/L ?
AP Chemistry
Mr. Markic
Page 10 of 12
Sample Exercise
Exactly 200 mL of 0.0040 M BaCl2 are added to exactly 600 mL of 0.080 M K2SO4. Will a precipitate form?
If 2.00 mL of 0.200 M NaOH are added to 1.00 L of 0.100 M CaCl2, will a precipitate form?
A solution contains 0.020 M Cl- ions and 0.020 M Br- ions. To separate the Cl- ions from the Br- ions, solid
AgNO3 is slowly added to the solution without changing the volume. What concentration of Ag+ ions (in mol/L)
is needed to precipitate as much AgBr as possible without precipitating AgCl?
The solubility products of AgCl and Ag3PO4 are 1.6 x 10-10 and 1.8 x 10-18, respectively. If Ag+ is added
(without changing the volume) to 1.00 L of a solution containing 0.10 mol Cl- and 0.10 mol PO4-3, calculate the
concentration of Ag+ ions (in mol/L) required to initiate
(a) The precipitation of AgCl
(b) The precipitation of Ag3PO4
AP Chemistry
Mr. Markic
Page 11 of 12
Review of Concepts
AgNO3 is slowly added to a solution that contains 0.1 M each of Br-, CO32-, and SO42- ions. Which compound
will precipitate first and which compound will precipitate last? (Use the Ksp of each compound to calculate
[Ag+] needed to produce a saturated solution.)
The Common Ion Effect and Solubility
The presence of a common ion decreases the solubility of the salt
Sample Exercise
• Calculate the solubility of silver chloride (in g/L) in a 6.5 x 10-3 M silver nitrate solution.
Calculate the solubility in g/L of AgBr in
(a) Pure water
(b) 0.0010 M NaBr
AP Chemistry
Mr. Markic
Page 12 of 12
Big Idea 6: Any bond or intermolecular attraction that can be formed can be broken. These two processes are in
dynamic competition, sensitive to initial conditions and external perturbations.
Learning Objectives: 1.20, 3.7, 6.11, 6.12, 6.13, 6.14, 6.15, 6.16, 6.17, 6.18, 6.19, 6.20, 6.21, 6.22, 6.23
Duration: Early April
Textbook Chapter: 16
Enduring Understanding
Essential Knowledge
6.C: Chemical equilibrium plays an important
role in acid-base chemistry and in solubility.
6.C.1: Chemical equilibrium reasoning can be used to describe the
proton-transfer reactions of acid-base chemistry.
6.C.2: The pH is an important characteristic of aqueous solutions
that can be controlled with buffers. Comparing pH to pKa allows
one to determine the protonation state of a molecule with a labile
proton.
6.C.3: The solubility of a substance can be understood in terms of
chemical equilibrium.
Learning Objectives
1.20 The student can design, and/or interpret data from, an experiment that uses titration to determine the
concentration of an analyte in a solution.
3.7 The student is able to identify compounds as Bronsted-Lowry acids, bases, and/or conjugate acid-base pairs,
using proton-transfer reactions to justify the identification.
6.11 The student can generate or use a particulate representation of an acid (strong or weak or polyprotic) and a
strong base to explain the species that will have large versus small concentrations at equilibrium.
6.12 The student can reason about the distinction between strong and weak acid solutions with similar values of pH,
including the percent ionization of the acids, the concentrations needed to achieve the same pH, and the amount of
base needed to reach the equivalence point in a titration.
6.13 The student can interpret titration data for monoprotic or polyprotic acids involving titration of a weak or
strong acid by a strong base (or a weak or strong base by a strong acid) to determine the concentration of the
titrant and the pKa for a weak acid, or the pKb for a weak base.
6.14 The student can, based on the dependence of Kw on temperature, reason that neutrality requires [H+] = [OH–]
as opposed to requiring pH = 7, including especially the applications to biological systems.
6.15 The student can identify a given solution as containing a mixture of strong acids and/or bases and calculate or
estimate the pH (and concentrations of all chemical species) in the resulting solution.
6.16 The student can identify a given solution as being the solution of a monoprotic weak acid or base (including
salts in which one ion is a weak acid or base), calculate the pH and concentration of all species in the solution,
and/or infer the relative strengths of the weak acids or bases from given equilibrium concentrations.
6.17 The student can, given an arbitrary mixture of weak and strong acids and bases (including polyprotic systems),
determine which species will react strongly with one another (i.e., with K >1) and what species will be present in
large concentrations at equilibrium.
6.18 The student can design a buffer solution with a target pH and buffer capacity by selecting an appropriate
conjugate acid-base pair and estimating the concentrations needed to achieve the desired capacity.
6.19 The student can relate the predominant form of a chemical species involving a labile proton (i.e.,
protonated/deprotonated form of a weak acid) to the pH of a solution and the pKa associated with the labile
proton.
6.20 The student can identify a solution as being a buffer solution and explain the buffer mechanism in terms of the
reactions that would occur on addition of acid or base.
6.21 The student can predict the solubility of a salt, or rank the solubility of salts, given the relevant Ksp values.
6.22 The student can interpret data regarding solubility of salts to determine, or rank, the relevant Ksp values.
6.23 The student can interpret data regarding the relative solubility of salts in terms of factors (common ions, pH)
that influence the solubility.








