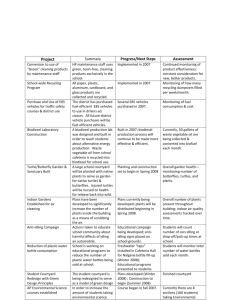
Project Proposal and Feasibility Study
advertisement

Project Proposal and Feasibility Study Team 14: GRE-cycle Hannah Albers, Ben Guilfoyle, Melanie Thelen, and Cole Walker ENGR 339--Senior Design Project December 8, 2014 1 Executive Summary The Senior Design team is designing a biodiesel production plant that uses triglyceride-containing restaurant waste oil and grease as its feed source. The proposed plant will use waste vegetable oil collected from local restaurants in Miami, FL and convert it to biodiesel. The main chemical reaction in this process is called transesterification. This reaction converts triglycerides in the feed to methyl esters (biodiesel) in the presence of an alcohol and a basic catalyst. The biodiesel produced will be compatible with current diesel engines. The demand for alternative energy sources is increasing as the demand for energy increases and global supply of fossil fuels decreases. Furthermore, restaurant grease is a waste product with no further applications, which makes it an ideal feedstock for biodiesel production. This design process prioritizes stewardship by recycling a waste product, caring by focusing on safety, and transparency by adhering to all government regulations on product quality. Many variables will be considered when designing this plant including: acid catalyst type, alcohol type, alkaline catalyst type and reactor type. For the purpose of this preliminary feasibility study, sulfuric acid, methanol, sodium hydroxide, and a batch reactor will be used. There are three main process sections: pretreatment, post-treatment and settling, with waste product distillation in between each. With these variables chosen, the project was found to be economically feasible. The initial construction costs were estimated to be $14.8 million. It was determined that a 10% rate of return is obtained when the selling price for biodiesel is $3.41. This falls in line with current biodiesel plant pricing. Rough preliminary cost estimates predict a yearly profit of $1.8 million would occur at this selling price, with a plant lifespan of 20 years. The customer will be fuel distributors with existing infrastructure to provide the public with blended biodiesel. Infinite demand will be assumed with potential competition coming from the diesel market. 2 Table of Contents Executive Summary ...................................................................................................................................... 2 Table of Contents .......................................................................................................................................... 3 Table of Figures ............................................................................................................................................ 6 Table of Tables ............................................................................................................................................. 7 1. Introduction ............................................................................................................................................... 8 1.1 Background Information ..................................................................................................................... 8 1.2 Objective ............................................................................................................................................. 9 1.3 Scope ................................................................................................................................................... 9 1.4 Past Project Teams ............................................................................................................................ 10 1.5 Feasibility.......................................................................................................................................... 10 1.5 Comparing Biodiesel and Petroleum Diesel ..................................................................................... 11 1.5.1 Differences in Chemistry ........................................................................................................... 11 1.5.2 Engine Modification .................................................................................................................. 12 1.5.1 Engine Performance ................................................................................................................... 12 2. Design Norms ......................................................................................................................................... 13 2.1 Stewardship ....................................................................................................................................... 13 2.2 Caring................................................................................................................................................ 14 2.3 Transparency ..................................................................................................................................... 14 3. Team Organization.................................................................................................................................. 15 Team Responsibilities ......................................................................................................................... 15 Hannah Albers .................................................................................................................................... 16 Ben Guilfoyle ...................................................................................................................................... 16 Melanie Thelen ................................................................................................................................... 16 Cole Walker ........................................................................................................................................ 16 4. Process Overview.................................................................................................................................... 17 4.1 Process Research ............................................................................................................................... 17 4.1.1 Block Flow Diagram .................................................................................................................. 17 4.1.2 Reaction Chemistry .................................................................................................................... 17 4.1.3 Key Variables............................................................................................................................. 19 4.1.4 Design Alternatives .................................................................................................................... 21 4.2 Material Research ............................................................................................................................. 22 4.2.1 Feed Sources .............................................................................................................................. 22 4.2.2 Feed Composition Research....................................................................................................... 23 4.2.2 Product ....................................................................................................................................... 25 3 5. Preliminary Design ................................................................................................................................. 26 5.1 Transesterification Reactor ............................................................................................................... 26 5.1.1 Mass Transfer Limitations ......................................................................................................... 26 5.1.2 Design Alternatives .................................................................................................................... 27 5.2 Catalyst ............................................................................................................................................. 32 5.2.1 Design Criteria ........................................................................................................................... 32 5.2.3 Design Alternatives .................................................................................................................... 32 5.2.4 Past Design Teams ..................................................................................................................... 33 5.2.5 Design Decision ......................................................................................................................... 34 5.3 Pre-Treatment Section ...................................................................................................................... 34 5.3.1 Filter ........................................................................................................................................... 34 5.3.2 Acid Treatment .......................................................................................................................... 36 5.3.3 Waste Separation........................................................................................................................ 38 5.4 Post-Treatment Section ..................................................................................................................... 38 5.4.1 Glycerin Separation.................................................................................................................... 38 5.4.2 Methanol Recovery .................................................................................................................... 41 6. Equipment ............................................................................................................................................... 43 6.1 Equipment Listing............................................................................................................................. 43 7. Safety Considerations ............................................................................................................................. 45 7.1 Chemicals.......................................................................................................................................... 45 7.2 Operating........................................................................................................................................... 46 8. Business Plan .......................................................................................................................................... 47 8.1 Market Study..................................................................................................................................... 47 8.1.1 Customer .................................................................................................................................... 48 8.1.2 Competition................................................................................................................................ 49 8.2 Tax Information ................................................................................................................................ 50 8.3 Costs.................................................................................................................................................. 51 8.3.1 Capital Costs .............................................................................................................................. 51 8.3.2 Operating Costs .......................................................................................................................... 51 8.4 Profitability ....................................................................................................................................... 52 9. Conclusion .............................................................................................................................................. 54 References ................................................................................................................................................... 55 Appendix ..................................................................................................................................................... 58 1. Overall Process Mass Balance ............................................................................................................ 58 2. Filter Calculations ............................................................................................................................... 59 4 3. Settler Calculations ............................................................................................................................. 60 4. Competing Biodiesel Plants in Florida ............................................................................................... 61 5. Material Safety Data Sheets (MSDS) ................................................................................................. 61 5 Table of Figures Figure 1: Biodiesel molecule ...................................................................................................................... 11 Figure 2: Petroleum Diesel Molecule ......................................................................................................... 11 Figure 3:Work Breakdown Schedule Organized as Critically Linked Tasks ............................................. 15 Figure 4: Overall Process Block Flow Diagram ......................................................................................... 17 Figure 5: Triglyceride Molecule ................................................................................................................. 18 Figure 6: Overall Biodiesel Reaction .......................................................................................................... 19 Figure 7: Saponification of Free Fatty Acids to form soap ......................................................................... 20 Figure 8:Water formation in an acid catalyst reaction to convert Fatty acids to biodiesel ......................... 20 Figure 9: Standard Curve of Linoleic Acid ................................................................................................. 24 Figure 10: TLC Plate of Butter, Linoleic Acid and Grease Extract ............................................................ 24 Figure 11:Transesterification of triglycerides to form biodiesel (methyl esters) ........................................ 26 Figure 12:Levenspiel Plot of the Pre-Treatment Reaction .......................................................................... 37 Figure 13:Materials of Construction for Handling Caustic Solution .......................................................... 44 Figure 14: Million barrels of biodiesel produced in the United States from January 2012 to May 2014 ... 48 Figure 15: Cash flow diagram for the plant lifespan................................................................................... 54 6 Table of Tables Table 1: EPA Biodiesel Specifications ....................................................................................................... 25 Table 2: Relative densities of effluent stream components ........................................................................ 39 Table 3: Equipment and Materials of Construction .................................................................................... 44 Table 4: Storage Vessel Volumes based on Storage Density Contents ...................................................... 45 Table 5: Current market value and anticpicated costs of process materials................................................ 52 7 1. Introduction 1.1 Background Information Fossil fuels account for approximately 82% of the United States’ energy supply. Though geologists estimate that less than half the total volume of crude in below-ground reserves will be depleted by 2030, it remains a fact that the supply of crude oil continues to decrease as the energy demand necessary to support the rapidly advancing lifestyles around the world increases. Oil has done well to advance human technology to the point where it is today, but the drawbacks of crude oil cannot be ignored. The demand for crude oil has caused wars, damaged the atmosphere, and eventually must be replaced by more sustainable energy sources. Used restaurant grease contains high levels of triglycerides, which store large amounts of energy. According to USA Today1, approximately 3 billion pounds of grease are produced in the United States each year. The average fast food restaurant produces about 150 - 200 pounds of grease every week, says the New York Times. The disposal of waste grease has been a large burden on restaurants, since grease cannot be processed in a wastewater treatment plant. It must be collected in a grease trap and disposed of in alternative ways. Restaurants recently have been selling their used grease to recycling companies that convert the used grease into fresh cooking oil. Rather than recycling the grease for consumption, the burden of grease waste disposal can be alleviated by instead converting this grease to fuel. Also, in contrast to fossil fuels, biofuels are produced from renewable plant and animal material such as vegetable oils, grease, or animal fats. Over the past decade, interest in producing biodiesel from oils and grease has grown into a marginally successful industry as the future availability of fossil fuels became uncertain. Additionally, burning biodiesel produces 56-87% less greenhouse gas emissions than conventional diesel2, which makes it a viable and promising option as an alternative to crude oil. 1 Ron, Barnett. "Restaurants' Grease a Hot Item for Thieves." USA Today 2 Beer, T, T Grant, and PK Campbell. "Biodiesel could reduce greenhouse gas emissions." CSIRO. CSIRO, 27 Nov. 2007. 8 1.2 Objective The main objective of this project is to design a biodiesel production plant that will provide a clean alternative to diesel. Petroleum production is unsustainable whereas the supply of restaurant grease is readily available. Obtaining feedstock for the plant will require the participation of restaurants, which currently have to pay an outside source to dispose of the grease they generate on a daily basis. By establishing the plant in a populous area with a lot of restaurants, a significant amount of biodiesel feedstock will be available. Secondly, the team purposes to make the plant profitable. Biodiesel production will not increase unless there is money to be made in the industry. Despite its environmental implications, production will be limited if it continues to be more economical to use fossil fuels to run our vehicles. By designing a profitable biodiesel plant, the team hopes to prove that biodiesel production is part of the answer for a more sustainable future. 1.3 Scope The team plans to design a production plant in the Miami area that will buy used grease from surrounding restaurants and convert it into pure biodiesel. The size of the Miami area will allow significant grease collection for the production of a substantial amount of biodiesel. Based on the number of restaurants in the area and the fact that grease will not be obtained from every one of these sources, the production of biodiesel is anticipated to be in the hundreds of barrels per day range. This comes from the estimation of available feed at 130,000 kg/day. This calculation can be found in section 4.3.1. Biodiesel is commonly blended with conventional diesel in 50:1, 20:1, or 5:1 diesel to biodiesel ratios. The team assessed the possibility of blending the produced biodiesel at the plant but determined that this was outside the scope of the project. The plant will be used exclusively to produce purified biodiesel that can be blended elsewhere. 9 1.4 Past Project Teams Several senior design teams have completed projects pertaining to biodiesel production. In 2001, Team FAME designed a plant that produced biodiesel in a continuous process for missionary transportation services in third world countries. In 2008, Team Rinnova designed a small scale biodiesel reactor for home users using feedstock from Calvin College Dining Services. Rinnova met their project goals but included recommendations for a second prototype. Suggested changes included adding a completely electronic control system, using better materials for piping, and installing a coarser filter. The Diesel Crew took these recommendations in 2013-2014 and designed a second prototype for home users. Their design utilized a microwave reactor, unlike the batch reactor designed by Rinnova, as the Diesel Crew was interested in designing a continuous system. Though the scope of this report includes a full-scale plant design, references to these project groups are found scattered throughout this report, particularly in the design alternatives for each plant section. The team will not be directly using other team projects by scaling up the past designs, but will instead take into consideration their design decisions while evaluating alternatives. 1.5 Feasibility The use of biodiesel compared to currently available diesel has both advantages and disadvantages. For one, restaurant grease can be obtained inexpensively, which makes the plant more economically feasible by lowering operating costs. See section BLANK for differences in operating costs between a petroleum plant and a biodiesel plant. Additionally, biodiesel has positive environmental implications as it produces less hydrocarbons and carbon monoxide than regular diesel when burned3. The proposed process is more sustainable by helping the environment and eliminating waste. The traditional diesel process requires a depletion of Earth’s natural resources to produce the fuel, while biodiesel uses otherwise useless waste as its feedstock. 3 "How Much Does Biodiesel Reduce Air Pollutants?" AllegroBiodiesel. 10 Despite these advantages, the use of biodiesel instead of traditional diesel does pose some challenges, most notably gelling. In colder weather, some biodiesels solidify into a gel that renders them unusable. This is one reason the plant will be placed in Florida. Considering the warm climate in the state, gelling should not be a concern. Though biodiesel significantly decreases carbon emissions, it is less efficient than normal diesel. It has been found that fuel efficiency is reduced by 10% compared to regular diesel4. Despite these drawbacks, the team feels that the economic and environmental considerations make the proposed process a valuable and feasible project to pursue. 1.5 Comparing Biodiesel and Petroleum Diesel 1.5.1 Differences in Chemistry Figure 1 depicts a typical biodiesel molecule. It is composed of a long carbon atom chain (average of 16-20 carbons) with an ester functional group, shown in blue, on one end. 5 Figure 1: Biodiesel molecule A typical petroleum diesel molecule looks very similar, except it does not have the same ester functional group attached to one end. 6 Figure 2: Petroleum Diesel Molecule The ester group on the biodiesel molecule makes the fuel much less toxic and more biodegradable than petroleum diesel. Enzymes such as esterase recognize the ester group on the biodiesel and begin fatty acid degradation7. This process breaks the molecule down to generate acetyl-CoA, which can be 4 "Biodiesel." fueleconomy.gov. US Department of Energy ”The Chemistry of Biodiesel”. Goshen College 6 ”The Chemistry of Biodiesel”. Goshen College 5 7 McKay, DB, “Degradation of triglycerides by a pseudomonad isolated from milk” 11 metabolized and thus is biodegradable and non-toxic. Biodiesel is safer for both the environment and human contact due to these characteristics. A biodiesel spill would be much less concerning than a petroleum diesel spill and since biodiesel can be metabolized, it is not as toxic to humans. 1.5.2 Engine Modification Due to the similarity in chemical structure, a diesel engine can run on biodiesel fuel with minor modification. Diesel engines manufactured pre-1993 may contain rubber tubing that may soften with biodiesel fuel and should be replaced with biodiesel-rated components. These are typically made of fluoro-elastomers like Teflon and will not degrade with biodiesel8. If the biodiesel fuel is a blend with less than 20% pure biodiesel, there is no need to replace the rubber tubing. There is most likely no need to replace the tubing on a car manufactured post-1993 either, but a mechanic should examine the engine before switching to biodiesel. Also, biodiesel is more viscous than petroleum diesel so it may gel, causing engine issues while trying to start the car9. In cool climates, engines may have to be modified by adding a fuel heating system or using a fuel additive that will lower the viscosity. Viscosity lowering fuel additives are available commercially, such as Wintron®, a Biodiesel Cold Flow Additive10. 1.5.1 Engine Performance Overall the engine performance using biodiesel is comparable to using petroleum diesel; however, some discrepancies occur in fuel efficiency and engine power. The energy content of biodiesel is slightly less than petroleum diesel, so the overall fuel efficiency is approximately 10% less. Also, engine power is reduced by 3 to 5% while using biodiesel since it has less energy per unit volume than petroleum diesel.11 “Using Biodiesel Fuel in Your Engine” Penn State Extension. “Engine Modification,” University of Strathclyde Engineering 10 ”Biodiesel Cold Fuel Additives,” Biofuel Systems Group LTD 11 “Using Biodiesel Fuel in Your Engine” Penn State Extension. 8 9 12 Engine clogging can occur if low-quality biodiesel is used since it will contain more deposits and viscous materials. However, this should not be an engine performance concern if high quality biodiesel is used. Also, care should be taken that the biodiesel does not oxidize and thus polymerize before it is burned, since this may clog a standard diesel engine. As long as the biodiesel is stored properly before being distributed, this should not be a major concern. Proper storage includes storing at moderate temperatures and not allowing the biodiesel to sit for extensive amounts of time before use.12 As mentioned in the introduction, engine emissions are also significantly reduced using biodiesel. Sulfur emissions are in effect non-existent and hydrocarbon emissions are reduced by an average of 50%13. The only trade-off is a slight increase in nitrogen oxide emissions. Nitrogen oxide (NOx) is a greenhouse gas and can cause smog and acid rain14. Overall though, the reduction in the carbon emissions so beneficial that the slight increase in NOx is negligible. 2. Design Norms Crucial to the sustainability of the proposed plant from both business and ethical standpoints is the promotion and prioritization of certain design norms, three of which are detailed in this report. These norms should be integrated into early planning stages of the plant and throughout the detailed design work, construction, and daily operation to ensure the health, well-being, and integrity of employees and customers alike. 2.1 Stewardship From its conception, the biodiesel plant proposal is centered on the importance of environmental stewardship. The proposed biodiesel plant would lower total emissions, generate less hazardous waste, and draw from a readily available and constant feed source of grease and turn something originally considered waste into a desirable product. Furthermore, it becomes increasingly vital in a world of “Using Biodiesel Fuel in Your Engine” Penn State Extension. “Biodiesel Emissions,” Biodiesel: America’s Advanced Biofuel. 14 ”Nitrogen Oxide,” U.S. National Library of Medicine. 12 13 13 unlimited demand and limited resources to think forward to a time when the nonrenewable resources currently used for energy generation are no longer a feasible option. Additionally, exercising stewardship requires examining the consequences of energy consumption and making every effort to mitigate the problems (greenhouse gas emissions or high volumes of dangerous waste material) faced by the energy industry. 2.2 Caring Once built, the proposed plant would require employees to operate the plant equipment. Caring, one of the design norms, becomes an important attribute of the design engineers for the safety of all employees. From the reactor catalyst selection to the layout of the plant units, caring must be exercised to ensure employees aren’t required to put themselves in harm’s way as part of their job description and to ensure safety procedures are established for routine and non-routine tasks. This design norm will be practiced when designing the controls used in the plant and by not introducing dangerous chemicals into the design without taking proper safety measures. 2.3 Transparency The potential for dangerous situations extending beyond the plant property are necessary to internally evaluate and communicate to local government and local citizens who could be negatively affected by the plant. Transparent business practices require full disclosure to employees and citizens of potential hazards, Furthermore, since the final product must meet certain governmental standards to be used in diesel engines, it is important to the team to ensure that the plant meet or even go beyond these standards. No shortcuts or half-measures will be taken in the design process in an attempt to promote the profitability of the design over the quality. 14 3. Team Organization To meet the team goal of designing a profitable plant, it was necessary to plan ahead. Task deadlines were assigned based on both class and team-specific deadlines. Professor Jeremy VanAntwerp was assigned to the team as a project advisor, and he met with the team every other week to discuss progress and design challenges. Professor VanAntwerp connected the team with Randy Elenbaas, who served as an industrial consultant. Randy Elenbaas is a chemical engineer employed at Vertellus Specialties, Inc. in Zeeland, Michigan with valuable knowledge of process design and project management. As seen in Figure 3, the project could be divided into three sections with research integrated throughout the process: the main reaction with pretreatment, the main reaction without pretreatment, and post-treatment. This schedule illustrates the team’s method of approach for choosing the most profitable design. Figure 3: Work Breakdown Schedule Organized as Critically Linked Tasks Team Responsibilities In addition to the tasks specified below, each team member was responsible for ongoing research about their section of the design and biodiesel production in general. This approach enabled all members to be familiar with all aspects of the project in order to validate and constructively critique each other’s 15 work. Deliverables for class deadlines such as the team poster and executive summary were produced collectively to ensure all team members were aligned on the objectives and scope of the project as these elements became more specific. Additionally, each team member was responsible for communicating their section of the design verbally and in writing relevant documents. Hannah Albers Hannah was tasked with the preliminary transesterification reactor design including catalyst aspects such as catalyst selection, reactor type, and preliminary sizing. She was also responsible for posttreatment research and recording the team’s weekly progress to be reported to Professor VanAntwerp during regular meetings. Ben Guilfoyle Ben was tasked with preliminary pretreatment design including esterification reaction kinetics, sizing, and design alternatives, and he was also responsible for feedstock research in the Miami area. Ben also worked in cost estimation and proving the plant’s profitability. He is the team’s webmaster and will keep the team’s website up to date throughout the year. Melanie Thelen Melanie was also tasked with preliminary transesterification reactor design such as reaction kinetics, sizing, and design alternatives. She headed research pertaining to governmental biodiesel standards and regulations and safety, and she kept the team organized by ensuring all class deadlines were met and deliverables were submitted on time. Cole Walker Cole was also tasked with the preliminary pretreatment design including filter selection and design alternatives. He was also responsible for financial research pertaining to market trends and plant profitability. Melanie and Cole are also working in the lab with grease samples to help the team better understand typical compositions of restaurant grease for the design. 16 4. Process Overview 4.1 Process Research 4.1.1 Block Flow Diagram Figure 4: Overall Process Block Flow Diagram 4.1.2 Reaction Chemistry The precursor to biodiesel molecules is a triglyceride, seen below 17 Figure 5: Triglyceride Molecule15 A triglyceride is an ester composed of three fatty acids connected with a glycerol backbone. The fatty acids in waste grease can either be saturated (animal fat derivative) or unsaturated (vegetable oil derivative). A glycerol molecule consists of three hydroxyl (HO-) groups, which form ester bonds with the carboxyl (-COOH) groups in the fatty acids. Triglycerides can be burned on their own in diesel engines but the three “tails” can become tangled, increasing the viscosity. A highly viscous fuel could congest fuel injectors and other engine internals. Instead, transforming triglycerides into biodiesel molecules will lower the viscosity. The main reaction that converts a triglyceride to biodiesel is called transesterification. In this process, triglycerides are contacted with methanol, which causes the single carbon-oxygen bonds to break. The methanol then reacts with the free end of the fatty acid to form a methoxy group, creating a biodiesel molecule. Since one triglyceride molecule “frees” 3 fatty acids, 3 molecules of biodiesel are produced for every one triglyceride. As shown in Figure 1, the final biodiesel molecule will have a long carbon chain with an ester group, formed by the addition of the methoxy group. The overall reaction is shown in Figure 6. 15 ”The Chemistry of Biodiesel”. Goshen College 18 Figure 6: Overall Biodiesel Reaction16 where R1, R2, R3 are alkyl groups which can be the same or different. When methanol breaks the carbonoxygen bonds in the triglyceride, the entire backbone molecule remains intact and becomes glycerol or soap. As seen in the chemical mechanism, a base catalyst is used this reaction and will be discussed in depth in section BLANK. 4.1.3 Key Variables The feedstock to be used in the process will be trap grease—that is food waste grease that is unable to be processed as wastewater. The oil portion of the grease may or may not be solid at room temperature but it will contain solid food particles that need to be filtered out. Within trap grease, there are two main types of grease: brown grease and yellow grease. Brown grease is essentially rotten food oil while yellow grease is rendered animal fat and used vegetable frying oil. The most significant difference in regard to biodiesel production is the free fatty acid (FFA) content of the grease. Brown grease contains more than 15% FFA while yellow grease has less than 15%. An increased amount of FFA is undesirable since it decreases the amount of triglycerides that can be turned into biodiesel. However, there are methods of turning FFAs into esters, which will be discussed later. Yellow and brown grease can either be processed simultaneously or separated beforehand. The rest of the grease is composed of triglycerides, which can be directly converted to biodiesel, as seen in Figure 3. The majority of processes involve a pre-treatment of high FFA feedstock with an acid catalyst. An acid catalyst, like sulfuric acid or hydrochloric acid, will convert the FFAs to esters, increasing the 16 Lasry, Sophie, “Renewable Energy” 19 overall conversion and product quality. It has been found that feed stocks need to have approximately 1% FFAs or less to provide acceptable product quality. One problem with pre-treatment is the formation of water as a byproduct of the FFA to ester reaction. This water needs to be removed using a separation technique since it would otherwise contaminate the final product. Another issue with the acid pretreatment is potential damage to vessels, so an appropriate material needs to be selected for the vessels. This acid pretreatment can be repeated to lower the FFA level to less than 1% since a lower FFA content indicates a higher overall process conversion to biodiesel. Additionally, FFAs can cause undesirable side reactions in the reactor, such as saponification. Saponification is a soap formation process, as seen in Figure 7. Figure 7: Saponification of Free Fatty Acids to form soap17 The FFA needs to be removed or converted before contacting with NaOH base to prevent the soap formation. The saponification reaction needs to occur in the presence of water, so the water formed in the acid catalyst reaction, seen in Figure 8, needs to be removed. Figure 8: Water formation in an acid catalyst reaction to convert Fatty acids to biodiesel 18 17 18 ”Biotechnology Trends,” Best Biotech ”Water Formation in Biodiesel Production,” Intech Journals 20 Despite pretreatment, some soap (glycerin, in this case) will inevitably form and pass through to the main reactor. Glycerin separation techniques will be discussed later in this report. Upon completing the pretreatment, the feed is transferred to the main reactor where it is heated to between 50°C and 60°C. The temperature must remain below the boiling point of the chosen alcohol so it remains in the liquid phase without pressurizing the reactor. A solution of alcohol and alkaline catalyst is prepared separately and added to the reactor. The alkaline catalyst is present to speed the reaction of triglycerides to methyl esters and convert any remaining FFA to soap. An agitator is used for up to one hour to ensure proper mixing, and the new solution settles. After proper separation, there will be a layer of biodiesel at the top of the reactor, and any soap formed will settle at the bottom. Once these layers are separated, biodiesel is ready for use. The variables to be manipulated in this process are: · · · · · · · · · Number of pre-treatment cycles (0, 1, or 2) Acid catalyst used in pre-treatment (sulfuric , hydrochloric , or other) Alcohol used in pre-treatment (methanol or ethanol) Ratio of acid catalyst to alcohol Temperature Alkaline catalyst (NaOH, KOH, NaOCH3, metallic Na, or other) Ratio of alkaline catalyst to alcohol Agitator time Settling Time It is important to note that this process assumes a given feedstock where the initial composition cannot be predicted or manipulated with accuracy. 4.1.4 Design Alternatives In addition to the process described above, there are many alternative processes to convert used grease to biodiesel under development. One option is using a supercritical reactor for processing grease with high FFA compositions. This process operates at high temperature (275°C to 325°C), high pressure, and therefore must take place in pressure-rated reaction vessels. This reactor does not require a catalyst because of the high temperature and pressure, so some separation steps are no longer necessary to purify the downstream product. Furthermore, side-reactions are less of a concern with no catalyst present. Initial FFA content, water formation and subsequent glycerol formation due to the high operating conditions less 21 affect the process. However, the capital and operating costs are very high. This is not a feasible option for a start-up plant of this scope. It would be better implemented as part of a revamp for a current facility since capital costs associated with pressure-rated vessels are higher. Another process is glycerolysis, a process that can handle feed stocks with greater than 10% FFA. Glycerin is heated to 400F and reacted with the FFAs in the feed to form monoglycerides. These monoglycerides can be processed as normal with the triglycerides by using an alkaline catalyst to form biodiesel. Problems with this process include high operating costs due to the high heat, a high-pressure boiler to keep the process in liquid phase, and a vacuum to remove any formed water. Glycerolysis is similar to the process mentioned earlier because the glycerin must be separated at the end, but there is much more glycerin produced in glycerolysis. Finally, the use of solid acid catalyst is an emerging option for processing grease. Solid acid catalyst is packed into a packed bed reactor and the mixture of grease and alcohol flows through the reactor. Water is still formed during the reaction though, so the alcohol/water solution separated the end must be distilled to recycle the alcohol. Also, contaminants in the oil like phosphorus and water can foul the catalyst. 4.2 Material Research 4.2.1 Feed Sources The feed grease will be collected from restaurants in the Miami area. In the Miami greater area, it was found that there are approximately 10,750 restaurants19. It was also found that an average restaurant produces about 35 lbs of grease per day20. This means that about 375,000 lbs of waste restaurant grease is produced per day in the Miami area. The greater Miami area is 6,137 square miles, which is well within reason to expect the plant’s trucks to be able to collect from the entire area. At the same time, it is not feasible to expect every restaurant to contribute to the plant’s feed stock, so it was estimated that the plant 19 20 Knight, Lauren. "How Many Restaurants and Bars are there in Miami?" Rosinski, Alan. "How Much Grease Fast Food Places Put Out." greener ideal 22 could collect approximately 75% of this waste grease. This leads to the estimation the plant will operate with a feed of 130,000 kg of waste restaurant grease per day. 4.2.2 Feed Composition Research In conjunction with BIOL383L, research was done to analyze the average composition of a sample of restaurant waste grease. The grease was obtained from Johnny's Café where they make both vegetable and animal products in the fryer. 4.2.2.1 Methods First, the waste grease was heated until homogenous and filtered through a cheesecloth to remove any suspended particles. Then, the grease was washed to reveal a lipid extract phase. A separatory funnel was used along with a chloroform-methanol solution to separate the lipid phase. Thin liquid chromatography (TLC) was used to analyze the lipid phase. This method uses a highpolarity silica coated plate and a low-polarity solvent that travels up the plate. A dot of the sample to be analyzed is placed at the bottom of the plate, and the plate is placed in a beaker with a small amount of solvent on the bottom. As the solvent travels up the plate, the movement of the sample depends on its polarity. Standards were created to compare the lipid sample movement to known compounds. Butter was used to simulate triglycerides and linoleic acid was used to simulate free fatty acids. Known concentrations of each of the standards and the lipid extract were run on a TLC column. Then the plates were stained with iodine chips to reveal the spots of sample. The area of the sample spot directly correlates to the concentration of the sample. 23 4.2.2.2 Results The area of the samples were calculated using a program called ImageJ, which counts the number of pixels in a traced area. Standard curves were generated which compared the known concentration to the number of pixels in the spot. Figure 9: Standard Curve of Linoleic Acid Then the extract was run and the area of the triglyceride spot and FFA spot were compared to the standard curves. Figure 10: TLC Plate of Butter, Linoleic Acid and Grease Extract 24 It was determined that this grease contains 29% FFAs. This agrees with literature values, which say that yellow grease contains 4-15% FFAs and brown grease contains 50-100% FFAs21 (). Yellow grease comes from vegetable oil and brown grease comes from animal fat. Since our sample contains both, 29% is a reasonable result and representative of waste grease from many restaurants that serve both animal and vegetable fried products. This value will be used to determine the amount of pre-treatment needed to reduce the FFA content to less than 1% (the standard for adequate conversion). 4.2.2 Product Table 1 features the federal EPA specifications for 100% biodiesel stock fuel that will be met. Table 1: EPA Biodiesel Specifications All of these specifications will be met via separation techniques. 21 ”Brown Grease Feedstock for Biodiesel.” National Renewable Energy Laboratory 25 5. Preliminary Design 5.1 Transesterification Reactor For the main reactor, three parts methanol and one part triglycerides are sent to a reactor for the transesterification of triglycerides to methyl esters according to the reaction presented below: Figure 11: Transesterification of triglycerides to form biodiesel (methyl esters) The catalyst in the reaction is typically a salt (typically sodium hydroxide or potassium hydroxide), which assumes that the waste vegetable oil has been pretreated to decrease the amount of FFAs by a processed described below. A broader range of catalysts types is considered to ensure maximum conversion. 5.1.1 Mass Transfer Limitations Mass transfer primarily limits the yield and purity of the biodiesel product. Triglycerides and methanol form two immiscible phases when combined, and the rate of reaction is directly influenced by the degree of mass transfer between phases. According to the Wilke-Change empirical model for diffusivity, the diffusivity coefficient between two liquid phases decreases with increasing viscosities, and highly viscous triglycerides impede diffusion into methanol. Increasing the interfacial area between phases can mitigate mass transfer limitations. Most reactors have some form of agitation such as impellers or baffles that use shear force to decrease the droplet size of methanol and triglycerides, increase the interfacial area of both phases. Mass transfer is greater in turbulent flow, which agitation promotes. Other methods include acoustic cavitation, or the 26 rapid growth and collapse of cavities in the reactor. When the cavities collapse, the liquid rushing into the preoccupied space creates shear forces that are capable of breaking chemical bonds of the triglyceride molecules and thus reducing droplet size. Alternatively, running the reaction above the supercritical temperature of methanol forces it to form a homogeneous phase with the triglycerides, but high energy costs to heat the reactor feed accompany this scenario. The use of multiple micro-reactors also promotes mass transfer. Interfacial area in micro-channels increases with decreasing micro-channel reactors, which promotes high conversion. All biodiesel reactor types either contain or can be modified to contain mass transfer promoting elements, but these characteristics also contribute to more difficult post-treatment, as smaller droplets are more stable when dispersed than larger droplets. Both mass transfer and subsequent separation must be considered in selecting a reactor. 5.1.2 Design Alternatives Selecting a reactor for optimal biodiesel production requires tradeoffs between high capital costs, reaction times, energy requirements, and conversion. Batch, semi continuous, and continuous reactors are most commonly used to produce methyl esters. 5.1.2.1 Batch Reactors Individuals who independently make biodiesel for personal use most commonly use batch reactors. They are easy to build for a small, garage-scale operation and have a low capital cost. Batch reactors can convert a wide variety of feedstocks into methyl esters, which is favorable when restaurant grease that, by nature, has a variable composition is used. The downsides to batch reactors are low conversion, high energy requirements, and long total reaction time. Batch reactions typically take place with either sodium or potassium hydroxide as a catalyst for transesterification, which is inefficient and leads to low yields. Research shows that adding 27 an agitator or series of agitators increases conversion up to 99% while decreasing reaction time. Agitator speeds typically range from 200-900 rpm, with higher speeds promoting greater conversion. Despite lowering reaction time, the combined time of heating, reacting, cooling, and preparing for subsequent runs makes the batch reactor time inefficient. Waste cooking oil must pass through a pretreatment step to lower FFA content in order to decrease the amount of glycerol formed as a side reaction during transesterification. This side reaction lowers methyl ester selectivity, and glycerol must be later separated from the methyl esters in order to meet production standards. 5.1.2.2 Continuous Reactors 5.1.2.2.1 Plug-Flow Reactors (PFRs) In a PFR, the reaction takes place inside a simple tube or pipe. PFRs are used in continuous processes with liquid or gas reactants and catalysts. Conversion increases with increasing residence time and volume. PFRs are rarely used in biodiesel production, according to the literature, because the length of the reactor necessary to achieve the desired conversion is often impractical 5.1.2.2.2 Packed Bed Reactors Packed bed reactors are essentially plug-flow reactors containing a solid catalyst. These reactors ensure an even mixing of reactants throughout the reaction tube to provide higher conversions than batch reactors but require several hours for this conversion. Activated carbon is typically the solid of choice coupled with oxides or alcohols. Long tube lengths for PBRs also tend to disqualify them as feasible reaction vessels. 5.1.2.2.3 Continuous Stirred Tank Reactors (CSTRs) CSTRs are vessels with agitation used in continuous processes. While a single CSTR does not outperform a batch reactor in terms of conversion when used in biodiesel production22, CSTRs in series 22 Mazubert, Alex, et al. Intensified processes for FAME production from waste cooking oil: A technological review 28 can approach high conversions. The expense of additional multiple reaction vessels tends to exclude CSTRs from popular usage. 5.1.2.2.4 Membrane Reactors Membrane reactors utilize a selectively permeable membrane for methyl ester conversion. Triglycerides enter the reactor on one side of the membrane while the methanol enters on the other. Unreacted mono-, di-, and triglycerides are contained on one side of the membrane as the reactants come into contact, which facilitates a simpler separation of triglycerides downstream of the reactor. Additionally, the membrane reactor can be designed to retain glycerols so that triglycerides with higher FFA content could be used. Most membranes are made out of carbon for low cost and ease of production, but they are also made of ceramic, polymers, silica, and zeolites. Membrane reactors have low capital and operating costs and can be used in continuous processes, however the time required for high conversion, similar to a batch reaction, is approximately 1-2 hours. 5.1.2.2.5 Micro-reactors Micro-reactors that consist of many small, parallel micro-channels have also been used in biodiesel production. A liquid catalyst such as sodium hydroxide is used to drive the transesterification that takes place simultaneously in all the channels. The reaction in a micro-reactor would take place near room temperature so there would be lower energy requirements than at elevated temperatures. Corrosion issues are avoided by constructing micro-reactors out of plastic resins such as polysulfone and polytetrafluoroethylene. Use of micro-reactors drastically decreases reaction time in comparison to batch reactions and other continuous reactors, but the pretreatment step cannot be avoided. High FFA content can cause plugging in the micro-channels. 5.1.2.2.6 Microwave Reactors Microwave reactors are used in batch and continuous processes. This type of reactor heats the reactants through microwave irradiation, which provides all the necessary heating and mixing. 29 Conversion with this heat effect can be over 97% with a residence time of less than one minute. The Diesel Crew built a microwave reactor for their small-scale biodiesel process, but it would be difficult to scale this process up to an industrial level since most home users aren’t converting high volumes of grease and thus utilize their personal microwaves. 5.1.2.2.7 Cavitational Reactors There are two different types of cavitational reactors—acoustic and hydrodynamic—that promote the biodiesel transesterification reaction by generating gaseous cavities that create high energy densities as the cavities grow and collapse. Cavities are created with pressure in acoustic cavitational reactors and a change in geometry in hydrodynamic cavitational reactors. These cavities also result in fine emulsions that increase mass transfer. When acoustic cavitational reactors were studied, residence times of less than one minute resulted in over 80-100% yields for transesterification at room temperature. Further separations are easier due to lower operating temperatures and smaller methanol-oil ratios. The difficulty with these reactors is similar to microwave reactors; they are troublesome to scale up for plant operations. 5.1.2.2.8 Oscillatory Baffled Reactor Oscillatory baffled reactors are essentially PFRs with baffles spaced evenly throughout the tube with an oscillating throughput flow rate. This flow rate facilitates recirculation of the reactants for increased residence time and greater mass and heat transfer while still maintaining liquids entering and exiting the reactor at steady state. OBRs can achieve 99% conversion in approximately 10 minutes, are compatible with homogeneous and heterogeneous catalysts, and are designed to recycle unreacted methanol. Methanol recovery is facilitated by larger droplet sizes leaving the reactor because adequate conversion can be achieved without adding extra agitation or other means to decrease the reactant droplet sizes. 30 5.1.2.2.9 Reactive Distillation In reactive distillation, the reaction and separation steps take place simultaneously in a single distillation column. Methanol and the triglycerides are combined in a single feed stream and drive the reversible reaction in favor of methyl ester conversion. Unreacted methanol exits from the top of the column while the methyl esters leave as bottoms products with a conversion of nearly 95%. The energy requirements for this process are high and the pretreatment step still necessary, but reactive distillation may be favorable if a distillation column is already necessary to separate waste water and methanol. 5.1.3 Past Design Teams Team FAME purposed to design a continuous process for their customers and selected two plugflow reactors with a glycerol separation following each. They assumed that this configuration would give a 98.2% yield of methyl esters. Team Rinnova designed a batch process that was accompanied with recommendations for a second prototype. The control system for the labor-intensive batch process required modifications. In light of this, the Diesel Crew designed a continuous process using a microwave reactor 5.1.4 Preliminary Design Decision A continuous process is desired over a batch process for multiple reasons. Batch processes require down time for the system to heat, cool, be cleaned, and prepared for the next reaction, which involves more controls and manpower to accomplish. If employees are required to assist with cleaning and loading the reactor, an additional safety risk is imposed on the overall system. Additionally, all the downtime steps introduced with batch systems present more opportunities for error. Batch processes are preferred over continuous processes when a larger degree of control over the system is required, but biodiesel production does not require this. While evaluating advantages and disadvantages of alternative reactors, the team narrowed the focus of the reactor design to only include design variables that most significantly increase the quality and 31 quantity of the desired product. The greatest limitation of the process is mass transfer, and the selected reactor must be equipped to mitigate this limitation without influencing the overall costs. The team will continue to research other reactor types to ensure that the best type is selected for our objectives. 5.2 Catalyst 5.2.1 Design Criteria The catalyst selected to promote transesterification must meet certain criteria, and one of the most important factors is cost. If, from the beginning, biodiesel is less profitable than diesel, lowering the cost of materials used will help increase profit margins and make the plant a worthwhile venture. Furthermore, the faster the transesterification proceeds, the more grease can be processed and sold. The catalyst should minimize undesirable side reactions, and a significant portion must be recycled in an energy efficient manner to further cut costs. 5.2.3 Design Alternatives Catalysts used for transesterification include acids, bases, clays, and enzymes. Bases are most commonly used largely because they are less expensive than clays and enzymes, and they promote faster reaction rates than acids. 5.2.3.1 Homogeneous Bases Homogeneous bases such as NaOH, KOH, and methoxides (reacting alkali bases with an alcohol such as methanol) are the most commonly used catalysts because they are inexpensive, promote high reaction rates, and are not accompanied by high energy requirements. However, these bases also react with FFAs to produce soaps, which decreases overall methyl ester yields and create emulsions that make product purification difficult. Alkali catalysts are also known to form soaps with water. Homogeneous catalysts tend to be less expensive and easier to use than heterogeneous catalysts. 32 5.2.3.2 Heterogeneous Bases Heterogeneous catalysts such as MgO, CaO, and NaOH on AlCl3 are easily separated from products. While homogeneous catalysts must either be disposed of or recycled after separation, a solid catalyst like CaO can be regenerated and reused easily. FFAs still react with heterogeneous catalysts to form soaps, and catalyst leaching becomes a further issue. Most heterogeneous bases are rendered ineffective when used at room temperature, so higher energy costs are necessary to protect the catalysts from degenerating. The primary drawback to solid catalysts is deactivation. The catalysts used can absorb some of the reactants and increasingly render catalytic sites useless. When this happens, the catalyst must either be regenerated or replaced with fresh catalyst, and both of these alternatives require time and added expenses. Recent studies indicate that strontium oxide (SrO), or SrO doped SiO2, as a catalyst promotes yields over 90% in approximately 10 minutes and has a FFA tolerance above the typical 3 wt%. SrO catalysts can tolerate between 3 and 3.5 wt% FFAs. However, SrO is more expensive than typical alcohol catalysts. 5.2.4 Past Design Teams Team Rinnova’s design utilized KOH over NaOH for transesterification because it mixes well with methanol. Once mixed with methanol, NaOH required a full day to dissolve, whereas KOH dissolves much faster. Liquid catalysts are ideal for batch reactions because the amount of catalyst needed is essentially the amount necessary for one batch. Team FAME also used KOH for the same reasons and also found that using KOH makes downstream separation easier. The Diesel Crew used CaO, a solid commonly sold by cement supply companies. One of their objectives was to design a continuous process, and solid catalysts lend themselves to this type of system. 33 5.2.5 Design Decision The team is leaning towards using NaOH because it is inexpensive, readily available, and most compatible with a batch reaction system. The cost difference between KOH and NaOH is marginal since the catalyst is already one of the least costly aspects of the design. The catalyst choice may be revisited in the future. 5.3 Pre-Treatment Section 5.3.1 Filter The waste vegetable oil (WVO) contains food debris and particulates that must be removed to prevent waste build up in the pumps and pipes and to control the quality of the feed entering the acid treatment. The pre-treatment filter must be able to remove the roughly 3% solids from the feed. 5.3.1.1 Leaf Filter: Vertical pressure leaf filters are applied for liquids with low solid content (roughly 1% to 7%). The vessel of the leaf filter can be suited with a steam jacket to control the temperature in the filter for liquids that require higher operating temperatures. Vertical pressure leaf filters can be used, rather than horizontal filters, in cases where light/fine particles are removed to minimize floor space. Vertical pressure leaf filters have applications in the fuel and biofuel industry to filter crude oil and fatty acids. The fact that these filters have been used in the industry previously ensures they would be a good choice for the plant. Also, the maximum filter area in a vertical leaf filter is approximately 100 m2. This should be noted, as multiple vertical leaf filters would be needed in this process to achieve the necessary filtration. The steam jacket capability of the vertical pressure leaf filter is essential to maintain a low viscosity of grease, meaning if this filter is chosen the steam jacket will most likely be necessary. 5.3.1.2 Centrifuge: Centrifuges are commonly used to separate solids from liquids. It uses centripetal acceleration to separate a mixture based on density; the denser substance moving farther from the axis of rotation, while 34 the lesser dense substance is being pushed toward the axis. Large industrial centrifuges are used to separate waste grease into white, yellow, and brown grease based on fatty acid concentration. It is also used in the biofuel industry for fuel purification. Using a centrifuge for the pretreatment would not only separate the solids from the grease, but also separate the grease into a heterogeneous mixture; yellow and brown grease. The centrifuge would most likely result in four separate phases: solids, brown grease, yellow grease, and water. The separation of the liquid phase would complicate the system. The different phases would have to be treated separately and the system would have to include two processes; a process to treat the yellow grease and a separate process to treat the brown grease. This would involve multiple pretreatments stages and reactors. To simplify the system and reduce capital costs, the team wishes to have a single homogeneous liquid phase for the grease feed. Therefore, the centrifuge would not be a desirable filtering method for the system. 5.3.1.3 Preliminary Design: Vertical leaf filters were selected to separate out the solids from the waste grease feed. This comes from the fact that the leaf filters are able to handle the expected solid content of the feed (3 wt %) as well as its wide use in filtering fatty acid mixtures. The vertical leaf filter can also be fitted with a steam jacket, which is necessary to maintain high temperature in the filter vessel and minimize grease viscosity. In order to size the filters needed the following equation was employed. LA(1-𝜀)𝜀p = cs(V+𝜀LA) In the above equation, L is the cake thickness, epsilon is the porosity of the cake, rho is the density of the solid particles in the cake, cs is the weight of solids per volume of filtrate, and V is the volume of filtrate. These are used to solve for A, or the filter area needed in the process. A complete calculation and the values used for the above properties can be found in Appendix 2. From this, it was 35 determined that 225 m2 of filter area would be needed for the process. As the maximum filter area of a vertical leaf filter is 100 m2, it was decided that 3-75 m2 vertical leaf filters will be used in the process. 5.3.2 Acid Treatment If a batch or micro-reactor is chosen to produce biodiesel, an acid treatment must proceed the transesterification reaction to lower the FFA content in the feed to less than 1%. However, this is not true if the catalyst used for transesterification is not an alcohol and thus will not form glycerol soaps in an undesirable side reaction. The design for this pre-treatment reactor will be covered, but, as stated above, will not be needed in some scenarios. In the treatment reactor, sulfuric acid is used as an acid catalyst. Methanol, in the presence of this acid, esterifies the FFAs. Methanol was chosen over ethanol as the pretreatment alcohol because it is more commonly used and is significantly cheaper than ethanol23. This not only increases the overall conversion in the main reactor, but also improves the quality of the biodiesel product by removing impurities. If the FFA content is above the 1% mark mentioned previously, the quality of the product will not be sufficient to meet regulations. It was determined that this acid treatment will take place in an isothermal PFR. This was selected by analyzing results from an experiment found in the Iraqi Journal of Chemical Engineering24. The isothermal PFR provides higher ester formation than an isothermal CSTR of the same volume. It is also is more efficient than any reactors run adiabatically. An isothermal batch system could provide the needed conversion will similar efficiency, however the drawbacks of a long set up and cleaning time leads to the selection of the PFR. Knowing the intended flow rates of each component in the plant through the process flow diagram, a preliminary volume of the pretreatment reactor was determined based on a simplified rate law. 23 24 "Ethanol and Unleaded Gasoline Average Rack Prices." State of Nebraska. 2014. Abbas, Ammar S., and Sura M. Abbas. "Kinetic Study and Simulation of Oleic Acid Esterification in Different Type of Reactors." IASJ. 36 For the preliminary design, it was assumed that the reaction follows an elementary rate law. This was modeled as follows; -rFFA = kCFFACMeOH. Further modifications were made to this rate law in order to express it in terms of conversion. The derivation led to the rate law being expressed as, -rFFA = kC2FFA,0(1-X)(𝜀-X). Here, 𝜀 refers to the ratio of methanol concentration to free fatty acid concentration entering the reactor. These initial concentrations were found by using molar masses and densities with initial molar flow rates. From this, it was found that the initial concentration of FFAs is 1.046 mol/L and the initial concentration of methanol is 24.71 mol/L. Through research it was found that typical k values in these types of reactions average about 0.0833 L/mol hr25. Using the values and equations outlined above, a Levenspiel plot was made which plotted conversion on the x-axis and the value of FFA molar flow rate divided by the reaction rate on the y-axis. This plot can be seen below: Figure 12: Levenspiel Plot of the Pre-Treatment Reaction 25 Barrios, M, J Siles, and A Martin. "A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil." 37 Using the process flow diagram it was determined that a conversion of 97% in the pre-treatment is needed to keep the FFA content entering the main reactor under 1%. The volume of the PFR needed is then equal to the area under the curve in from a conversion of 0 to 0.97. This area was calculated by first fitting a polynomial equation to the Levenspiel plot. This polynomial was then integrated and solved using the above limits to find the necessary volume of 460 L. The preliminary recommendation is then to use a 460 liter PFR to produce the needed FFA concentration entering the main reactor. 5.3.3 Waste Separation The stream leaving the pretreatment reactor is comprised of waste water, methanol, triglycerides, and trace amounts of FFAs. Water must be removed from the stream before it enters the transesterification reactor. Otherwise, the water and FFAs will react to form glycerin and lower biodiesel selectivity. The most effective separation technique is distillation. The difference in volatility between water and triglycerides make distillation an effective separation technique. Though distillation is accompanied with high energy requirements, this rigorous separation will ensure the water leaves the stream accompanied by methanol (which has a higher volatility) and does not interfere with the transesterification process. A system mass balance shows that methanol in the water stream after pretreatment accounts for less than 1% of the total volume, so another distillation column would not be necessary to separate water and methanol. 5.4 Post-Treatment Section The products leaving the main reactor are primarily methyl esters, excess methanol, and glycerin. The objective of the post-treatment section is two-fold: the methyl esters must be purified to meet government specifications, and the excess methanol must be recovered and fed back to the main reactors. 5.4.1 Glycerin Separation The EPA specifies that up to 0.24 wt% glycerin can be present in biodiesel product. Even with a rigorous pretreatment process to mitigate soap generation, glycerin in the reactor effluent must be 38 separated from the biodiesel, and one way to accomplish this is adding a large settling tank downstream of the reactor. The glycerin will enter the tank with the liquid products, and settle to the bottom. A settling tank would be simple and effective because other components in the effluent stream are much less dense, as shown in Table 2, and the product flowing out of the tank would have only trace amounts of glycerin. Table 2: Relative densities of effluent stream components Effluent Stream Density (g/L) Glycerin 1.26 Methanol 0.792 Methyl Esters <1 Triglycerides (unreacted) <1 The team was advised to avoid a solid-liquid separation. Solids are usually avoided in industry as they are harder to work with than liquids. The glycerin is a liquid when leaving the reactors, but has a melting point near room temperature (64 F). As this is still a lower temperature, the team finds it wise to add some form of heat exchange to ensure glycerin remains a liquid. The most obvious alternative is to heat the stream entering the settling tank. This could take the form of a heat exchanger immediately after the reactors, or reactors operating at elevated temperatures. Keeping this heating precaution in mind, a large tank is the most likely settling device used to remove glycerin. A preliminary sizing of this settling tank was conducted. For these calculations, a more simplified example of a biodiesel and glycerin mixture was examined. The methanol was neglected because it has the lowest density and therefore is not expected to interfere with the separation between the methyl esters and glycerin. The first step in sizing the tank is to determine the dispersed phase. To do this, the equation 39 is employed. Here, Q refers to volumetric flow rates, which are gathered from the process flow diagram, ρ refers to fluid densities and µ refers to fluid viscosities. The subscript l is a reference to the light phase and h is to the heavy phase. As indicated previously, in this case the biodiesel is the light phase and glycerin is the heavy phase. This equation produces a θ of 7.83, which means the heavy phase, or glycerin, is dispersed26. Precise values for these variables can be found in Appendix 3, along with a full calculation of the settling tank sizing. Viscosities were taken at 25 degrees Celsius as this slightly raised temperature ensures liquid phase is maintained. After determining the dispersed phase, it is necessary to decide which settling law applies. The following equation was used to do this. Above, subscript f refers to the biodiesel and subscript p refers to the glycerin. The variable dp is the average droplet size of the dispersed phase, glycerin, which forms as it falls out of mixture. It was determined from literature research that this diameter is best approximated as 70 µm27. When this value is used in the above equation, Kc is found to be equal to 0.38. For Kc values less than 3.3 and droplet sizes between 3-100 µm, Stoke’s Law is used to find the settling velocity28. It is also important to know that flow into the settler must be laminar to utilize Stoke’s Law, a condition that will be maintained when the piping is designed in detail. 26 Seader, Henley, and Roper. Separation Process Principles. 3rd ed. 795. Abeynaike, A, AJ Sederman, Y Khan, ML Johns, and JF Davidson. "The experimental measurement and modelling of sedimentation and creaming for glycerol/biodiesel droplet dispersions." Malcolm Mackley. Chemical Engineering Science 28 Seader, Henley, and Roper. Separation Process Principles. 3rd ed. 793. 27 40 As mentioned above, Stoke’s Law allows for the calculation of the settling velocity of the mixture. This can then be used to size the tank. Stoke’s Law expresses From this equation, the settling velocity, ut was calculated to 4.19 x 10-4 m/s. This value must be equal to or larger than the quotient of biodiesel volumetric flow and interface area. The interface area is estimated as the diameter of the tank times its length. A ratio for length to diameter of 5 is used, which is taken as a guideline from references29. This leads to the derivation of the equation The minimum diameter is then calculated to 0.77 m. A safety factor is added, resulting in the preliminary design of a 1 m tank diameter. Using the length to diameter ratio, the final preliminary dimensions of the settling tank are 5 meter long by 1 meter in diameter. 5.4.2 Methanol Recovery 5.4.2.1 Design Criteria The EPA specification for methanol is less than 0.2 vol% in the finished biodiesel product. The team is currently researching different methods of separating methanol and methyl esters, which include distillation, centrifugation, and pervaporation. Centrifugation is discussed in the pretreatment section of this report and will not be described in detail here. 29 Seader, Henley, and Roper. Separation Process Principles. 3rd ed. 794. 41 5.4.2.2 Design Alternatives 5.4.2.2.1 Distillation Methanol and water are commonly separated by distillation. The Diesel Crew utilized Team Rinnova’s vacuum distillation design, which requires an operating pressure below 1 atm, and found it effective for their purposes. There is over a 60°F difference between the boiling points of water and methanol, which makes distillation an attractive and effective choice. However, distillation is the generally the most energy intensive separation technique, and the added energy costs of operating a vacuum tower in a plant may make a different separation technique more feasible. 5.4.2.2.2 Pervaporation Pervaporation is a technology that is commonly used to separate water from organic solvents. The process takes advantage of differences in polarity and molecular size to pass a smaller and more polar molecule through a selective membrane that is aided by a vacuum. The membrane used is inert, and the only energy requirements are heating the incoming stream (the process is more effective at high temperature) and running the vacuum. Since methanol and water are similar in their polarity, the membrane selection would be particularly important. One downside to pervaporation is that it is usually precedes a distillation column and may not be effective as the only method of separation. If distillation or a centrifuge will be required following pervaporation, it may be more worthwhile to use the budget for one very effective column or centrifuge. 5.4.2.2.3 Design Decision A distillation column will ensure maximum separation of methanol and water and is the best alternative. Equilibrium data for this binary mixture is readily available in the literature at several different temperatures, and UNISIM simulation software will be used in the future to model the characteristics and energy requirements of the column. 42 6. Equipment 6.1 Equipment Listing The Table 3 describes the equipment and materials of construction necessary for the process. Carbon steel is a standard material of construction because of its affordability and commonality in the chemical industry. In units where corrosion is a concern due to acids or bases, such as the sulfuric acid and potassium hydroxide in our process, more corrosive resistant materials must be considered. The esterification plug flow reactor and pre-treatment distillation column will contain significant amounts of sulfuric acid. For these vessels, carbon steel coated with stainless steel inner coating was selected as the best material. The mixer and transesterification reactor come into contact with small amounts of potassium hydroxide. Figure 13 was used to determine the materials of construction for the mixer and transesterification reactor. The figure uses the sodium hydroxide as a base, which is more basic that potassium hydroxide. Therefore, any material suitable for sodium hydroxide would also be suitable for potassium hydroxide, using the same concentration and temperature conditions. No more than 4% potassium hydroxide is expected to exist within the vessels at any point of the process and the temperature of the solution will be about 60°C. Under these conditions, the solution is located in Area A using the figure, indicating that carbon steel is a suitable material for the mixer and transesterification reactor. 43 Figure 13: Materials of Construction for Handling Caustic Solution30 Table 3: Equipment and Materials of Construction Equipment Vertical Leaf Filters (3) Esterification Plug Flow Reactor Pre-Treatment Distillation Column Mixer Transesterification Reactor Post-Treatment Distillation Column Settling Tank Material Of Construction Carbon Steel Stainless Steel coating Stainless Steel coating Carbon Steel Carbon Steel Carbon Steel Carbon Steel Further equipment will be necessary to ensure necessary energy is supplied and removed from the system to achieve the desired volume of product. This equipment will include, but is not limited to, heat exchangers, pumps, valves, and vessels. The number of vessels and respective volumes are shown in 30 "Caustic Soda Solution Storage Tank Lining." Dow Answer Center. The Dow Chemical Company, 6 Nov. 2014. Web. 07 Dec. 2014. 44 Table 4. The appropriate quantity and energy requirements of other necessary equipment will be determined once additional design decisions are made, the most important of which is the main reactor. Table 4: Storage Vessel Volumes based on Storage Density Contents *denotes vessels that are incorporated into the process but are not represented in the process flow diagram Vessel Contents Feed Grease Methanol Sulfuric Acid Post Pre-Treatment* Glycerin Biodiesel Wastewater* KOH Volume (m3) 5 5 50 0.5 150 15 150 3 0.5 Material of Construction Carbon Steel Carbon Steel Stainless Steel Carbon Steel Carbon Steel Carbon Steel Carbon Steel Stainless Steel 7. Safety Considerations 7.1 Chemicals The main chemicals used in this process that present safety concerns are the acid catalyst, the base catalyst and the alcohol. For this preliminary design and safety evaluation sulfuric acid, potassium hydroxide and methanol will be used. The Material Safety Data Sheets for these chemicals can be found in Appendix 5. Sulfuric Acid is a colorless, odorless, and highly-corrosive material. The main acute exposure hazard is severe burns to skin and eyes. It is more harmful than other strong acids due to the dehydrating nature of the chemical, which releases extra heat, causing secondary burns. It will cause temporary or permanent blindness if contacted with eyes in either liquid or vapor form. Long term exposure also causes lung damage, vitamin deficiency and potentially cancer. Potassium Hydroxide is a caustic base and white solid that is typically available in flakes or pellets. It is a highly corrosive alkali that will decompose living tissue on contact. It also causes secondary burns, as the decomposition reaction is highly exothermic. Aqueous potassium hydroxide is more dangerous than solid, although solid KOH will also exhibit some corrosive behavior if there is any water 45 present (including sweat or humid air). There are no known long-term exposure effects of KOH; all of the health effects are acute effects due to corrosivity. Methanol is a colorless, flammable liquid with a distinct odor. If ingested, methanol will be metabolized to formic acid, which damages the central nervous system and causes blindness, coma or death. The adverse health effects associated with methanol all occur internally. While contact with skin will not cause external damage, it may provide a route for the chemical to enter one’s central nervous system. Methanol is highly flammable and easily ignites. 7.2 Operating Sulfuric acid must be stored in a vessel made of a non-reactive reactive material, such as glass. Great care should be taken that the acid does not contact the operator’s skin. Proper personal protective equipment (PPE) for handling sulfuric acid includes: safety goggles, face shield, boots, gloves and aprons made from a suitable material (see MSDS for more information). Sulfuric acid will be pumped directly from the storage vessel to the pre-treatment vessel, limiting the amount of operator contact needed. If sulfuric acid does contact the skin though, any contaminated clothing must be removed and the affected person must wash the acid off under a safety shower for at least 15 minutes. Then seek medical attention immediately. Also, when diluting the sulfuric acid the acid must be added to the water instead of water added to the acid. This way, the high heat capacity of water will absorb the heat released as the two chemicals mix. Potassium hydroxide must also be stored in a non-reactive vessel, preferably the container in which it was delivered. Keep sealed tightly in a cool, dry, well-ventilated area. When handling potassium hydroxide, the same PPE should be worn as for sulfuric acid. If an operator needs to create the potassium hydroxide solution, a respirator should also be worn. The same procedure as for the sulfuric acid should also be followed if potassium hydroxide contacts skin. 46 Methanol must be stored in a cool, dry, well-ventilated area away from any potential sparks. If the methanol does ignite, water will not extinguish the fire. A fire extinguisher will be necessary. Methanol will be pumped directly from a storage container to the various vessels, so operator contact with methanol is limited. If an operator must come into contact with the methanol, the same PPE as for sulfuric acid must be worn. If the area is not well ventilated, a respirator must also be worn. If methanol is ingested, drink two glasses of water and seek medical attention immediately. If methanol contacts any part of the body, follow the same procedure as for sulfuric acid. All three chemicals are considered hazardous waste and need to be properly disposed of according to OSHA standards. 8. Business Plan 8.1 Market Study Despite being a relatively new energy source with oldest plants dating back to the mid-2000s, biodiesel is a growing market in the United States. According to the US Energy Information Administration, approximately 100 million gallons of biodiesel per month are produced in 96 US plants that have a combined capacity of 2 billion barrels every year. Biofuels provide nearly 6% of the energy supplied annually, and as of 2009 the United States produces the second largest volume of biofuels. depicts the amount of biofuel produced monthly. It can be see that the volume steadily increases overall as more biodiesel plants are built every year. 47 Figure 14: Million barrels of biodiesel produced in the United States from January 2012 to May 2014 Producing biodiesel is profitable due to a $1 per barrel tax credit for blenders. Without this incentive, expensive feed and raw materials prevent biodiesel from competing with conventional diesel prices. Some states provide further benefits to biodiesel producers, as detailed in a following report section. 8.1.1 Customer The final product will be marketed and sold to blenders who have the capacity to blend large volumes of purified product and who will ultimately supply the blended fuel to consumers with diesel engines. These customers would potentially be community leaders, retail gas station owners, who value sustainability and desire to promote alternative energy sources. In keeping with the project objective of introducing a competitive product into the market, the target customer is one who would benefit from cheaper diesel. 48 The customer can further be expanded to include any industry using equipment powered by diesel that desires to use a greener energy source for their operations. Several such industries are power companies that use diesel for electricity generation, the sugar industry that uses diesel to fuel sugar boiler vessels, paint companies for wood preservatives, and the agricultural industry for aerial spraying. Ultimately, any company interested in biodiesel would be interested from an environmental standpoint or out of a desire to promote themselves as an energy-conscious company. The plant will be designed to operate for 20 years. At the end of the period the team will decide, depending on the market, to either sell the plant or specific pieces of equipment at salvage value for further profits or refurbish the equipment to continue biodiesel production for an extended period. In the scenario of retiring the plant, future customers would include those who have interest in producing biodiesel themselves or interest in a similar process. 8.1.2 Competition The competition in the biodiesel market comes from inside the biodiesel industry and the alternate fuel industries. The competition from the biodiesel industry comes from similar biodiesel production plants in the area. For the team, this competition pertains to the biodiesel production plants in Florida, who will compete for the business of the diesel blenders. According to the plants listing section of biodiesel.org, there are currently five companies in Florida that produce biodiesel. These business will need to sell their product to blenders who will then blend the biodiesel with diesel made from crude oil. The blended product is what will be sold to the public as biodiesel at the pump. There is also competition from the industries creating fuel other than biodiesel. The most comparative competition comes from the diesel industry because, biodiesel is compatible with diesel engines. If there is a large discrepancy between the price of biodiesel and diesel, the environmental benefits of biodiesel will not be enough to overcome the financial burden. This would result in the public 49 being unwilling to buy biodiesel versus regular diesel at the pump and consequently blenders will lose interest in purchasing biodiesel from the production businesses. This is unlikely because crude oil is a limited resource and trends show the price of diesel only increasing in the future. Therefore, the cost difference of biodiesel and diesel will continue to decrease until biodiesel becomes more cost effective than diesel. A more likely source of competition comes from the alternative fuel industry. There is a large effort to find alternative fuels such as electricity, wind, water, etc. Today vehicles either run on combustion engines, electric engines, or a combination of both. The biodiesel industry depends on the continual use of combustion engines. However, as electric motors become more efficient, combustion engines might eventually become obsolete. With so much research invested into alternative fuels, it is difficult to tell how the market will change in the future which is a main reason why the expected plant life is only 20 years. 8.2 Tax Information Under federal law, a $1 per-gallon tax credit will be applied for the production of biodiesel that complies with fuel standards and Clean Air Act requirements. This credit will be increased to $1.10 for the first 15 million gallons produced (approximately BLANK years of production). It is estimated that the production facility will receive tax credit for 100% of taxes with the 75% state rebate and $1.10/gallon rebate from the federal government. This credit is valid through 2017 and is expected to be renewed further. A major factor in determining the location of the plant was the tax credits available in certain states beyond the standard $1-per-barrel credit. Overall, Florida is the most helpful for renewable fuel plant start-ups and provides the proposed plant with the greatest chance of being competitive with diesel providers. 50 “An income tax credit is available for 75% of all capital, operation, maintenance, and research and development costs incurred in connection with an investment in the production, storage, and distribution of biodiesel (B10-B100), ethanol (E10-E100), or other renewable fuel in the state, up to $1 million annually per taxpayer and $10 million annually for all taxpayers combined.” This tax credit makes biodiesel production possible, since the process is not efficient enough to make a profit on its own. This credit is good through December 31, 2018. The credit may only be applied toward taxes; no rebates will be issued. 8.3 Costs 8.3.1 Capital Costs An efficient way to estimate the capital cost of the plant this early into the design process was put forward by Don Hofstrand of Iowa State University32. The cost including working capital is estimated by $1.57 of the nameplate capacity, or the gallons per year output of the plant. From the process flow diagram, it was found that an estimated 9.5 million gallons per year of biodiesel will be produced. After multiplying by the above cost factor, it can be preliminarily estimated that the total upfront cost of building the plant will be $14.8 million. 8.3.2 Operating Costs In a similar fashion to estimating capital costs, a rough estimate for operating costs can be made. Fixed costs can be estimated as $0.25 of the nameplate capacity and variable costs can be estimated as $0.26 of the nameplate capacity. Using the yearly production of 9.5 gallons per year as detailed previously it was calculated that the overall operating costs are preliminarily estimated at $4.8 million per year. 32 Hofstrand, Don. "Tracking Biodiesel Profitability." Iowa State University. 51 8.4 Profitability In order to determine the profitability of the proposed plant, a selling price for the biodiesel product to produce a rate of return of 10% was calculated. At a 10% return rate, the project becomes economically feasible. The first step in doing this was to calculate the costs of the needed chemical inputs. The costs for each of the process materials used can be found in the table below. It was determined, preliminarily, that potassium hydroxide will be used instead of sodium hydroxide due to its lower cost. A very small percentage of hydroxide is used compared to the feed stock, however, so the price difference becomes insignificant. If it is determined later that sodium hydroxide will be more beneficial to the process this switch can be easily made with minimal impact to the plant’s yearly costs. Table 5: Current market value and anticipated costs of process materials Material Cost/Unit Capacity $/yr Waste Grease $0.40/gal 58,987,500 gal/yr $ 23,595,000.00 Methanol33 $0.22/lb 8,895,893.74 lb/yr $ 2,490,850.25 Potassium Hydroxide34 $0.185/lb 393,250 lb/yr $ 72,751.25 Sulfuric Acid (95%)36 $0.18/lb 523,809 lb/yr $ 94,285.71 The totals costs per year then, including operating costs calculated previously, are $31.1 million. The total revenue of the plant comes from the sale of biodiesel and the side product glycerin. The glycerin can be sold for $0.10 per pound39, or a total of $0.74 million. The revenue of the biodiesel is not known 33 Methanex, 2014. https://www.methanex.com/our-business/pricing "Caustic Potash." ICIS. ICIS Chemical Business, 2006. 36 ICIS Chemical Business, Sulfuric acid market seeks balance, 2010. http://www.icis.com/resources/news/2010/09/06/9390780/sulfuric-acidmarket-seeks-balance/ 39 Ahmad, S, D Papadias, and Rick Farmer. "Hydrogen From Glycerol: A Feasibility Study." Hydrogen Energy. 34 52 at this point until the cash flow diagram is created. This process will described next and allows for a biodiesel selling price to be calculated. The cash flow diagram was created assuming a construction time of one year. For this reason the entire capital cost was placed in year -1 of the diagram. As stated above a 20 year life span was assumed as this is a typical plant life. So, the salvage price of the plant, 10% of the capital costs, and the working capital was added to year 20 of the cash flow diagram. Using this knowledge equations were used to bring all costs back to present value. For the plant construction cost using the necessary 10% return, the equivalent cost was found with, $14.8E6(1+0.10)1. Similarly the salvage and working capital were brought to present value using the 10% rate of return and the equation, $2.8E6(1+0.10)-20. The yearly profit can be solved for with the following equation and Microsoft Excel Solver, Yearly Profit((1+0.10)20-1)/(0.10(1+0.10)20) = $2.8E6(1+0.10)-20 - $14.8E6(1+0.10)1. Using the above equation it was found that the plant would need a yearly profit of $1.9 million in order to achieve the needed rate of return. This value was used with the prices of the other materials in the system detailed above to find a needed yearly biodiesel revenue of $32.2 million. Knowing the yearly output of biodiesel (9.5 million gallons), this can be converted to a selling price per gallon. In order for the plant to be economically feasible, then, the biodiesel will need to be sold for $3.41 per gallon. According to research this falls well within the reasonable selling price for biodiesel plants, meaning that the proposed process will be profitable. The complete cash flow diagram that had been described previously can be seen below. 53 Figure 15: Cash flow diagram for the plant lifespan 9. Conclusion In conclusion, it is feasible to open a 9.5 million gallon per year biodiesel production plant. While this plant alone will not solve the energy crisis, it can be used to demonstrate profitability for others looking to open biodiesel production plants. With preliminary estimates, the plant will produce a 10% rate of return over a 20 year lifespan. Many specific decisions still need to be made, such as the reactor type, but this report clearly outlines the main design criteria and options. Decision matrices will need to be formed to determine the best variable choices for this plant. These decisions should be made to further increase the overall rate of return while not infringing on any of the selected design norms. As these specific decisions are made next semester, the team will focus on more rigorous design work. 54 References Abbas, Ammar S., and Sura M. Abbas. "Kinetic Study and Simulation of Oleic Acid Esterification in Different Type of Reactors." IASJ. Iraqi Journal of Chemical and Petroleum Engineering, June 2013. Web. 15 Oct. 2014. <http://www.iasj.net/iasj?func=fulltext&aId=75022>. Abeynaike, A, AJ Sederman, Y Khan, ML Johns, and JF Davidson. "The experimental measurement and modeling of sedimentation and creaming for glycerol/biodiesel droplet dispersions." Malcolm Mackley. Chemical Engineering Science, n.d. Web. 5 Dec. 2014. Ahmad, S, D Papadias, and Rick Farmer. "Hydrogen From Glycerol: A Feasibility Study." Hydrogen Energy. N.p., 2010. Web. 2 Nov. 2014. <http://www.hydrogen.energy.gov/pdfs/progress10/ii_a_3_ahmed.pdf>. Alexander, Adam, Michael Lubben, Angus Richeson, and Thomas Voss. "Senior Design Final Report." Team 1: The Diesel Crew. Ed. Adam Alexander. Calvin College, 15 May 2014. Web. 1 Oct. 2014. <http://www.calvin.edu/academic/engineering/2013-14team1/Documents/Senior%20Design%20Final%20Report4.pdf>. Barrios, M, J Siles, and A Martin. "A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil." FUEL(2006): 2383-88. Web. 7 Nov. 2014. Barnett, Ron. "Restaurants' Grease a Hot Item for Thieves." USA Today. USA Today, n.d. Web. 4 Oct. 2014. <http://usatoday30.usatoday.com/news/nation/2010-09-29-restaurant-greasethieves_N.htm>. Beer, T, T Grant, and PK Campbell. "Biodiesel could reduce greenhouse gas emissions." CSIRO. CSIRO, 27 Nov. 2007. Web. 6 Dec. 2014. <http://www.csiro.au/OrganisationStructure/Divisions/Energy-Technology/BiodieselBlends.aspx>. "Biodiesel." fueleconomy.gov. US Department of Energy, n.d. Web. 16 Oct. 2014. <http://www.fueleconomy.gov/feg/biodiesel.shtml>. Buasri, Achanai, et al. "Transesterification of waste frying oil for synthesizing biodiesel by KOH supported on coconut shell activated carbon in packed bed reactor."ScienceAsia 38 (2012): 28388. Engineering Research Database. Web. 13 Oct. 2014. Buasri, Achanai, Bussarin Ksapabutr, Manop Panapoy, and Nattawut Chaiyut. "Biodiesel production from waste cooking palm oil using calcium oxide supported on activated carbon as catalyst in a fixed bed reactor." Korean Journal of Chemical Engineering 29.12 (2012): 1708-12. SciFinder Scholar. Web. 13 Oct. 2014. Buasri, Achanai, Nattawut Chaiyut, Vorrada Loryuenyong, Chao Rodklum, Thechit Chaikwan, and Nanthakrit Kumphan. "Continuous Process for Biodiesel Production in Packed Bed Reactor from Waste Frying Oil Using Potassium Hydroxide Supported on Jatropha curcas Fruit Shell as Solid Catalyst." Applied Sciences 2.3 (2012): 641-53. SciFinder Scholar. Web. 29 Oct. 2014. Canter, Neil. "Making Biodiesel in a Microreactor." Tribology & Lubrication Technology 62.8 (2006): 15-7. ProQuest. Web. 29 Oct. 2014. "Caustic Potash." ICIS. ICIS Chemical Business, 3 June 2006. Web. 4 Nov. 2014. <http://www.icis.com/resources/news/2006/06/03/2014563/caustic-potash/>. 55 Chen, Ching-Lung, et al. "Biodiesel Synthesis Via Heterogeneous Catalysis using Modified Strontium Oxides as the Catalysts." Bioresource technology 113 (2012): 8-13. ProQuest. Web. 4 Nov. 2014. Chilakpu, K. O., et al. "Modification of Biodiesel Batch Reactor." Journal of Emerging Trends in Engineering and Applied Sciences 5.4 (2014): 262-4. ProQuest. Web. 29 Oct. 2014. "Ethanol and Unleaded Gasoline Average Rack Prices." State of Nebraska. Official Nebraska Government Website, 1 Dec. 2014. Web. 6 Dec. 2014. <http://www.neo.ne.gov/statshtml/66.html>. "Fossil Fuels." Institute for Energy Research. IER, 2014. Web. 4 Nov. 2014. <http://instituteforenergyresearch.org/topics/encyclopedia/fossil-fuels/>. Harbert, Joshua, Christian Ocier, Mitch Kenyon, Fred Thielke, and Adebo Alao. "Final Report-Senior Design Project." Rinnova Renewable Energy. Ed. Joshua Harbert. Calvin College, 7 May 2008. Web. 31 Oct. 2014. <http://www.calvin.edu/academic/engineering/seniordesign/SeniorDesign07-08/Team11/downloads/Final_Report.pdf>. Hofstrand, Don. "Tracking Biodiesel Profitability." Iowa State University. ISU, n.d. Web. 6 Nov. 2014. <http://www.extension.iastate.edu/agdm/energy/html/d1-15.html>. Hosseini, Mehdi, Ali Mohammad Nikbakht, and Meisam Tabatabaei. "Biodiesel Production in Batch Tank Reactor Equipped to Helical Ribbon-like Agitator." Modern Applied Science 6.3 (2012): 40-45. ProQuest. Web. 29 Oct. 2014. "How Much Does Biodiesel Reduce Air Pollutants?" AllegroBiodiesel. N.p., 2007. Web. 6 Oct. 2014. <http://www.allegrobiodiesel.com/howmuchdoesbiodieselreduceairpollutants.html>. Irwin, Scott. "Pricing of 2014 Biodiesel RINs under Alternative Policy Scenarios." Farmdoc Daily. Department of Agriculture and Consumer Economics, University of Illinois Urbana, 15 Oct. 2014. Web. 9 Nov. 2014. <http://farmdocdaily.illinois.edu/2014/10/pricing-of-2014-biodieselrins.html>. Ito, Takuya, Yusuke Kakuta, Katsumi Hirano, and Toshinori Kojima. "Study on Continuous Production of Biodiesel Using Fixed Bed Reactors Filled With Anion-Exchange Resins." Energy and Environmental Research 4.2 (2014): 47-54.Engineering Research Database. Web. 1 Nov. 2014. Knight, Lauren. "How Many Restaurants and Bars are there in Miami? ." shiftgig. N.p., Dec. 2012. Web. 15 Sept. 2014. <http://www.shiftgig.com/articles/how-many-restaurants-and-bars-are-theremiami>. Kyte Centrifuge. N.p., n.d. Web. 9 Nov. 2014. <http://www.kytecentrifuge.com/applications/biodiesel>. Martin, Norbert. "Removal of Methanol by Pervaporation." Sulzer Technical Review. Sulzer Chemtech, 2003. Web. 8 Nov. 2014. <http://www.sulzer.com/ms//media/Documents/Cross_Division/STR/2003/2003_01_19_martin_e.pdf>. Mazubert, Alex, Martine Poux and Joelle Aubin. Intensified processes for FAME production from waste cooking oil: A technological review. 26 July 2013. PDF. "Member Plants Listing - Biodiesel.org." Member Plants Listing - Biodiesel.org. N.p., n.d. Web. 09 Nov. 2014. <http://www.biodiesel.org/production/plants/plants-listing>. "Miami Metropolitan Area." Wikipedia. Wikipedia, n.d. Web. 9 Oct. 2014. <http://en.wikipedia.org/wiki/Miami_metropolitan_area>. 56 Neji, Soumaya Bouguerra, Mahmoud Trabelsi, and Mohamed H. Frikha. "Esterification of Fatty Acids with Short-Chain Alcohols Over Commercial Acid Clays in a Semi-Continuous Reactor." Energies 2.4 (2009): 1107-17. ProQuest. Web. 29 Oct. 2014. "Renewable Energy." Institute for Energy Research. IER, 2014. Web. 4 Nov. 2014. <http://instituteforenergyresearch.org/topics/encyclopedia/fossil-fuels/>. Rosinski, Alan. "How Much Grease Fast Food Places Put Out." greener ideal. N.p., 25 June 2013. Web. 30 Sept. 2014. <http://www.greenerideal.com/business/0624-restaurant-grease-being-tolen/>. Saqib, Muhammad, Muhammad Waseem Mumtaz, Asif Mahmood, and Muhammad Imran Abdullah. "Optimized Biodiesel Production and Environmental Assessment of Produced Biodiesel." Biotechnology and Bioprocess Engineering 17 (2012): 617-23. Engineering Research Database. Web. 6 Nov. 2014. Seader, , Henley, and Roper. Separation Process Principles. 3rd ed. N.p.: John Wiley & Sons Inc., 2011. 792-95. Print. "USDA Livestock, Poultry & Grain Market News." USDA Agricultural Marketing Service. USDA, 7 Nov. 2014. Web. 7 Nov. 2014. <http://www.ams.usda.gov/mnreports/lswagenergy.pdf>. "Vertical Leaf Filter." Vertical Leaf Filter. N.p., n.d. Web. 09 Nov. 2014. <http://www.solidliquidseparation.com/pressurefilters/verticalleaf/verticalleaf.htm>. "Vertical Pressure Leaf Filter." , Vertical Leaf Filter, Pressure Leaf Filter, Leaf Filters Manufacturer & Supplier in Navi Mumbai, Used for Filtration of Crude Oil. N.p., n.d. Web. 09 Nov. 2014. <http://www.sharplex.com/vertical-pressure-leaf-filter.htm>. Vicente, Gemma, Mercedes Martinez, and Jose Aracil. "Integrated biodiesel production: a comparison of different homogeneous catalysts systems." Bioresource Technology 92 (2003): 297-305. Elsevier. Web. 9 Nov. 2014. 57 Appendix 1. Overall Process Mass Balance 58 2. Filter Calculations LA(1 - ε)ρp = cs (V + εLA) Cake thickness (L): estimated to 1cm Porosity (ε): estimated at 0.5 Volume of filtrate (V): used PFD and molar flows/density to find a volumetric flow of 145 m^3/day Weight of solids per volume of filtrate (cs): weight of solids = (3 wt %)(130,000 kg/day) cs=26.85 kg/m^3 Density of Solid Particles in Cake: used an average cake density of 3500 kg/m^3 Used in above equation to find Area of Filtration Needed per Day: 225 m^2 59 3. Settler Calculations First, determine the dispersed phase using: 𝑄𝑙 𝜌 𝜇 𝜃 = ( ) [ 𝑙 ℎ⁄𝜌ℎ 𝜇𝑙 ]0.3 𝑄ℎ Ql – Biodiesel Volumetric Flow Rate – 1.255x10-3 m3/s from PFD Qh – Glycerin Volumetric Flow Rate – 9.144x10-5 m3/s from PFD 𝜌𝑙 – Biodiesel Density - 875 kg/m3 𝜌ℎ – Glycerin Density - 1260 kg/m3 𝜇𝑙 – Biodiesel Viscosity – 4.5x10-3 Pa.s 𝜇ℎ – Glycerin Viscosity – 1.0x10-3 Pa.s It was determined from this that θ = 7.83, meaning glycerin is dispersed. Next the settling law to be used was determined with: (𝜌𝑓 )(𝜌𝑝 − 𝜌𝑓 ) (1) 𝐾𝑐 = 34.81𝑑𝑝 [ ]3 𝜇𝑓2 dp – glycerin droplet size falling from mixture – 70 µm from Literature (Densities and viscosities are same as above with subscript f referring to biodiesel and p to glycerin.) These values were converted to American Engineering Units, which are needed for the equation, resulting in a Kc value of 0.385. This means the Stoke’s Equation is applicable. The Stoke’s equation is, 60 𝑢𝑡 = 𝑔𝑑𝑝2 (𝜌𝑝 − 𝜌𝑓 ) 18𝜇𝑓 All values are the same as ones used above. These are used to solve for ut, or settling velocity. 𝑢𝑡 = 4.19𝑥10−4 𝑚/𝑠 Setting a constraint of L/D=5, and knowing interface area (A) = DL, the following equation was derived, 𝑄𝑏𝑖𝑜𝑑𝑖𝑒𝑠𝑒𝑙 ≥ 𝑢𝑡 5𝐷 2 Minimum D is found to be 0.77 m. Design suggested: D = 1m & L = 5m 4. Competing Biodiesel Plants in Florida 5. Material Safety Data Sheets (MSDS) See attached 61