ENGINEERING MATERIALS Engineers have to know the best and
advertisement

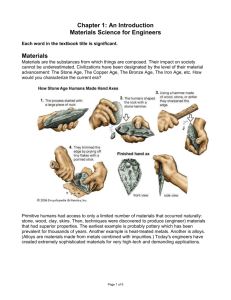
ENGINEERING MATERIALS Engineers have to know the best and most economical materials to use. Engineers must also understand the properties of these materials and how they can be worked. Most engineering materials fit into a few big, general categories. Metals We can divide metals into ferrous and non-ferrous. The former contain iron and the latter do not contain iron. Cast iron and steel, which are both alloys, or mixtures of iron and carbon, are the most important ferrous metals. Steel contains a smaller proportion of carbon than cast iron. Certain elements can improve the properties of steel and added to it. For example, chromium may be included to resist corrosion and tungsten to increase hardness. Aluminium, copper and the alloys (bronze and brass) are common non-ferrous metals. Plastics and Ceramics Plastics and ceramics are non-metals; however plastics can be machined like metals. Plastics are classified into two types – thermoplastics and thermosets. Thermoplastics can be shaped and reshaped by heat and pressure but thermosets cannot be reshaped because they undergo chemical changes as they harden. Ceramics are often employed by engineers when materials which can withstand high temperature are needed. Ceramics can be used to create bone and tooth replacements, super-strong cutting tools, or to conduct electricity. With the addition of oxygen or nitrogen, metals become ceramics, too. Semiconductors One of semiconductors - silicon - is making it possible for you to read something on the screen of your computer! That's because silicon is the essential material in an electronic computer chip. "Semiconductor" means a material can conduct electricity with a bit of help in the form of added "impurities." Your CD, DVD player, and telephone - all depend on semiconductors. Polymers Polymers are just very big molecules made of smaller molecules linked together into long, repeating chains. Rubber bands are made of polymers, so are paints and every kind of plastic. And by the way, most of the food we eat is made of natural polymers! Biomaterials Every part of our body is a material! Bone, muscles, fingernails, hair, and skin are all examples of different types of materials found in our body with remarkable properties that help us survive - from keeping us upright, and protecting us from heat or cold, to cutting and grinding our food. Some scientists try to mimic nature's designs to create materials for other uses, such as using the foam structure of bone as an inspiration for designing materials that are lightweight and strong. In the past, people used and changed materials by trial and error. And they worked on a big, visible scale - for example, heating then rapidly cooling chunks of iron to make it harder. Modern materials scientists manipulate and change materials based on fundamental understandings of how the materials are put together, often on the invisibly-tiny scale of atoms. Composite materials A composite material is made by combining two or more materials – often ones that have very different properties. The two materials work together to give the composite unique properties. The first modern composite material was fibreglass. It is still widely used today for boat hulls, sports equipment, building panels and many car bodies. The biggest advantage of modern composite materials is that they are light as well as strong. By choosing an appropriate combination of matrix and reinforcement material, a new material can be made that exactly meets the requirements of a particular application. Composites also provide design flexibility because many of them can be moulded into complex shapes. The disadvantage is often the cost. Although the resulting product is more efficient, the raw materials are often expensive. VOCABULARY PRACTICE 1. Read the following web page (1 –7) and complete the missing headings using the words in the box. Aluminium Copper Glass Plastic Rubber Steel Timber Recyclable Materials 1. __________ Scrap can be sorted easily using magnetism. If the metal is galvanized (coated with zinc) the zinc is fully recyclable. If it is stainless steel, other metals mixed with the iron, such as chromium and nickel, can also be recovered and recycled. 2. ___________ Sorting is critical, as there are key differences between the clear and coloured material used in bottles and jars, and high-grade material used in engineering applications, which contains traces of metals. 3. ___________ Scarcity makes recycling especially desirable, and justifies the cost of removing insulation from electric wires, which are a major source of scrap. Pure metal can also be recovering from alloys derived from it, notably brass (which also contains quantities of zinc, and often lead) and bronze (which contains tin). 4. ___________ The cost of melting down existing metal is significantly cheaper than the energy-intensive process of electrolysis, which is required to extract new metal from ore. 5. ____________ Hard wood and softwood can be reused. However, the frequent need to remove ironmongery and saw or plane off damaged edges, can make the process costly. 6. ____________ Tyres are the primary source of recyclable material. These can be reused in certain applications. They can also be ground into crumbs which have varied uses. 7. ____________ An obstacle to recycling is the need to sort waste carefully. While some types can be melted down for reuse, many cannot, or result in a low-grade material. 2. Match the materials from the web page (1-8) to the definitions (a-h). 1. stainless steel a a metal used to make brass, and in galvanized coatings on steel 2. zinc b the predominant metal in steel 3. iron c a type of steel not needing a protective coating, as it doesn’t rust 4. bronze d a dense, poisonous metal 5. lead e rocks from which metals can be extracted 6. hardwood f an alloy made from copper and tin 7. ore g timber from pine trees 8. softwood h timber from deciduous trees Discuss the following questions: 1. 2. 3. 4. 5. 6. 7. 8. What are metals divided into? What do ferrous metals contain of? What are the most important ferrous metals? What types are plastics classified into? Can thermosets be reshaped? Why? When are ceramics employed by the engineers? What does “semiconductor” mean? What are polymers? Discuss the following questions: 1. 2. 3. 4. 5. 6. 7. 8. What are metals divided into? What do ferrous metals contain of? What are the most important ferrous metals? What types are plastics classified into? Can thermosets be reshaped? Why? When are ceramics employed by the engineers? What does “semiconductor” mean? What are polymers? Discuss the following questions: 1. 2. 3. 4. 5. 6. 7. 8. What are metals divided into? What do ferrous metals contain of? What are the most important ferrous metals? What types are plastics classified into? Can thermosets be reshaped? Why? When are ceramics employed by the engineers? What does “semiconductor” mean? What are polymers? Discuss the following questions: 1. 2. 3. 4. 5. 6. 7. 8. What are metals divided into? What do ferrous metals contain of? What are the most important ferrous metals? What types are plastics classified into? Can thermosets be reshaped? Why? When are ceramics employed by the engineers? What does “semiconductor” mean? What are polymers? Discuss the following questions: 1. 2. 3. 4. 5. 6. 7. 8. What are metals divided into? What do ferrous metals contain of? What are the most important ferrous metals? What types are plastics classified into? Can thermosets be reshaped? Why? When are ceramics employed by the engineers? What does “semiconductor” mean? What are polymers?