Chapter 15 - Chemical Equilibrium
advertisement
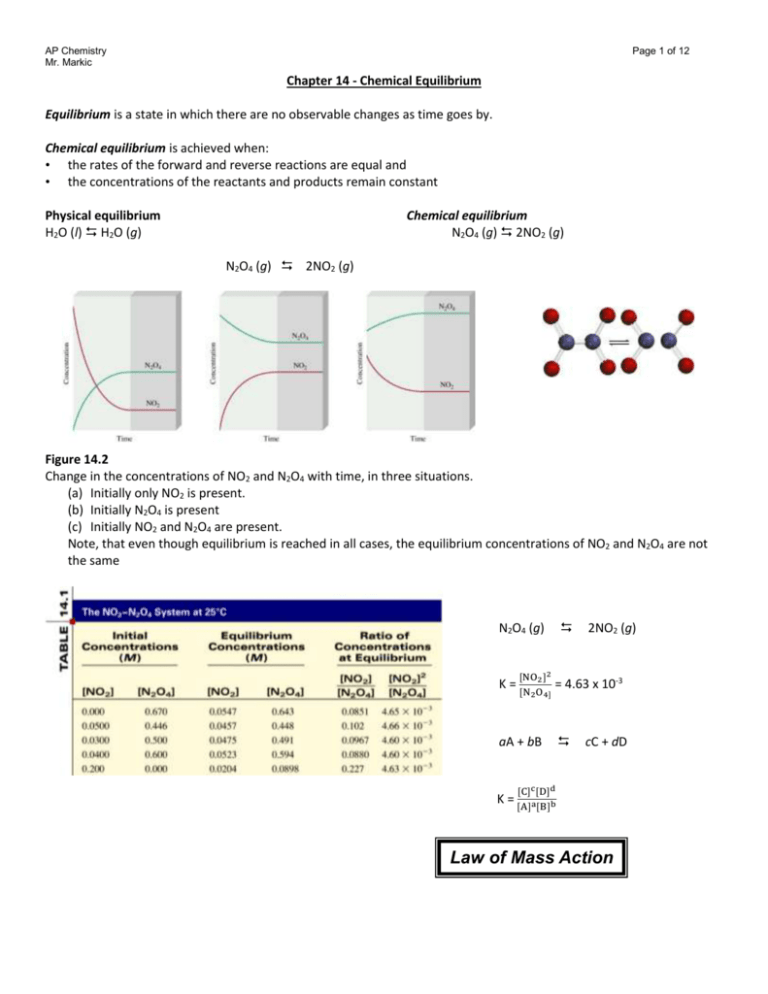
AP Chemistry Mr. Markic Page 1 of 12 Chapter 14 - Chemical Equilibrium Equilibrium is a state in which there are no observable changes as time goes by. Chemical equilibrium is achieved when: • the rates of the forward and reverse reactions are equal and • the concentrations of the reactants and products remain constant Physical equilibrium H2O (l) H2O (g) Chemical equilibrium N2O4 (g) 2NO2 (g) N2O4 (g) 2NO2 (g) Figure 14.2 Change in the concentrations of NO2 and N2O4 with time, in three situations. (a) Initially only NO2 is present. (b) Initially N2O4 is present (c) Initially NO2 and N2O4 are present. Note, that even though equilibrium is reached in all cases, the equilibrium concentrations of NO2 and N2O4 are not the same N2O4 (g) K= [NO2 ]2 [N2 O4] = 4.63 x 10-3 aA + bB K= 2NO2 (g) cC + dD [C]c [D]d [A]a [B]b Law of Mass Action AP Chemistry Mr. Markic Page 2 of 12 Equilibrium Will K >> 1 K << 1 Lie to the right Lie to the left Favor products Favor reactants Homogenous equilibrium applies to reactions in which all reacting species are in the same phase. N2O4 (g) 2NO2 (g) [NO2 ]2 2 O4] PNO2 2 Kc = [N Kp = 𝑃 N2 O4 In most cases Kc Kp aA (g) + bB (g) cC (g) + dD (g) Kp = Kc(RT) n ∆n = moles of gaseous products – moles of gaseous reactants = (c + d) – (a + b) Homogeneous Equilibrium CH3COOH (aq) + H2O (l) CH3COO- (aq) + H3O+ (aq) Kc ’ = [CH3 COO− ][H3 O+ ] [CH3 COOH][H2 O] [H2O] = constant Kc = [CH3 COO− ][H3 O+ ] [CH3 COOH] = Kc’ [H2O] General practice not to include units for the equilibrium constant. Sample Exercise Write the expressions for KC, and KP if applicable, for the following reversible reactions at equilibrium: (a) HF(aq) + H2O(l) H3O+(aq) + F-(aq) (b) 2NO(g) + O2(g) 2NO2(g) (c) CH3COOH(aq) + C2H5OH(aq) CH3COOC2H5(aq) + H2O(l) AP Chemistry Mr. Markic Practice Exercise Write Kc and Kp for the decomposition of dinitrogen pentoxide. Page 3 of 12 2NO(g) ↔ 4NO2(g) + O2(g) Sample Exercise The following equilibrium process has been studied at 230°C. 2NO(g) + O2(g) 2NO2(g) In one experiment the concentrations of the reacting species at equilibrium are found to be [NO] = 0.0542 M, [O2] = 0.127 M, and [NO2] = 15.5 M. Calculate the equilibrium constant (Kc) of the reaction at this temperature Practice Exercise Carbonyl chloride (COCl2), also called phosgene, was used in World War I as a poisonous gas. The equilibrium concentrations for the reaction between carbon monoxide and molecular chlorine to form carbonyl chloride CO(g) + Cl2 ↔ COCl2(g) At 74°C are [CO] = 1.2 x 10-2 M, [Cl2] = 0.054 M, and [COCl2] = 0.14 M. Calculate the equilibrium constant (Kc). The equilibrium constant KP for the decomposition of phosphorous pentachloride to phosphorous trichloride and molecular chlorine PCl5(g) PCl3(g) + Cl2(g) Is found to be 1.05 at 250°C. If the equilibrium of the partial pressures of PCl5 and PCl3 are 0.875 atm and 0.463 atm, respectively, what is the equilibrium partial pressure of Cl2 at 250°C? The equilibrium constant Kp for the reaction: 2NO2(g) ↔ 2NO(g) + O2(g) Is 158 at 1000 K. Calculate 𝑃𝑂2 if 𝑃𝑁𝑂2 = 0.400 atm and 𝑃𝑁𝑂 = 0.270 atm. AP Chemistry Mr. Markic Page 4 of 12 Methanol (CH3OH) is manufactured industrially by the reaction: CO(g) + 2H2(g) CH3OH(g) The equilibrium constant (Kc) for the reaction is 10.5 at 220°C. What is the value of KP at this temperature? For the reaction: N2 (g) + 3H2 (g) ↔ 2NH3(g) KP is 4.3 x 10-4 at 375°C. Calculate Kc for the reaction. Heterogenous equilibrium applies to reactions in which reactants and products are in different phases. CaCO3 (s) CaO (s) + CO2 (g) Kc ’ = [CaO][CO2 ] [CaCO3 ] Kc = [CO2] = Kc’ x [CaCO3 ] [CaO] Kp = PCO2 [CaCO3] = constant [CaO] = constant The concentration of solids and pure liquids are not included in the expression for the equilibrium constant. CaCO3 (s) CaO (s) + CO2 (g) PCO2 = Kp PCO 2 does not depend on the amount of CaCO3 or CaO Sample Exercise Write the equilibrium constant expression Kc, and Kp if applicable, for each of the following heterogeneous systems (a) (NH4)2Se(s) 2NH3(g) + H2Se(g) (b) AgCl(s) Ag+(aq) + Cl-(aq) (c) P4(s) + 6Cl2(g) 4PCl3(l) Write the equilibrium constant expression for Kc and Kp for the formation of nickel tetracarbonyl, which is used to separate nickel from other impurities: Ni(s) + 4CO(g) ↔ Ni(CO)4(g) AP Chemistry Mr. Markic Page 5 of 12 Consider the following heterogeneous equilibrium: CaCO3(s) CaO(s) + CO2(g) At 800°C, the pressure of CO2 is 0.236 atm. Calculate (a)Kp and (b) Kc for the reaction at this temperature Consider the following equilibrium at 395 K: NH4HS(s) ↔ NH3(g) + H2S(g) The partial pressure of each gas is 0.265 atm. Calculate Kp and Kc for the reaction. Review of Concepts For which of the following reactions is Kc equal to Kp? (a) 4NH3(g) + 5O2(g) ↔ 4NO(g) + 6H2O(g) (b) 2H2O2(aq) ↔ 2H2O(l) + O2(g) (c) PCl3(g) +3NH3(g) ↔ 3HCl(g) + P(NH2)3(g) A + B C + D Kc ’ [C][D] [E][F] Kc’ = [A][B] Kc’’ = [C][D] C + D E + F Kc’’ [E][F] A + B E + F Kc Kc = [A][B] so Kc = Kc’ x Kc’’ If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. N2O4 (g) 2NO2 (g) [NO2 ]2 2 O4] K = [N = 4.63 x 10-3 2NO2 (g) N2O4 (g) [N2 O4] K’ = [NO 2 2] 1 =K = 216 When the equation for a reversible reaction is written in the opposite direction, the equilibrium constant becomes the reciprocal of the original equilibrium constant. AP Chemistry Mr. Markic Page 6 of 12 Sample Exercise The reaction for the production of ammonia can be written in a number of ways: (a) N2(g) + 3H2(g) 2NH3(g) (b) 1 2 3 N2(g) + 2 H2(g) NH3(g) (c) 1 3 N2(g) + H2(g) 2 3 H3(g) Write the equilibrium constant expression for each formulation. (Express the concentrations of the reacting species in mol/L.) (d) How are the equilibrium constants related to one another? Practice Exercise Write the equilibrium expression (Kc) for each of the following reactions and show how they are related to each other: 2 (a) 3O2(g) ↔ 2O3(g) (b) O2(g) ↔ O3(g) 3 Review of Concepts From the following equilibrium constant expression, write a balanced chemical equation for the gas-phase reaction. Kc = [𝑁𝐻3 ]2 [𝐻2 𝑂]4 [𝑁𝑂2 ]2 [𝐻2 ]7 Writing Equilibrium Constant Expressions • The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. • The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. • The equilibrium constant is a dimensionless quantity. • In quoting a value for the equilibrium constant, you must specify the balanced equation and the temperature. • If a reaction can be expressed as a sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. Chemical Kinetics and Chemical Equilibrium 𝐾𝑓 A + 2B ↔ AB2 Kr Rate = kf [A][B]2 Rate = kr [AB2] ratef = rater 𝑘𝑓 𝑘𝑟 [AB ] 2 = Kc = [A][B] 2 kf [A][B]2 = kr [AB2] Review of Concepts The equilibrium constant (Kc) for the reaction A ↔ B + C is 4.8 x 10-2 at 80°C. If the forward rate constant is 3.2 x 102 s-1, calculate the reverse rate constant. AP Chemistry Mr. Markic Page 7 of 12 The reaction quotient (Qc) is calculated by substituting the initial concentrations of the reactants and products into the equilibrium constant (Kc) expression. IF • Qc > Kc system proceeds from right to left to reach equilibrium • Qc = Kc the system is at equilibrium • Qc < Kc system proceeds from left to right to reach equilibrium Sample Exercise At the start of the reaction, there are 0.249 mol N2, 3.21 x 10-2 mol H2, and 6.42 x 10-4 mol NH3 in a 3.50-L reaction vessel at 375°C. If the equilibrium constant (Kc) for the reaction N2(g) + 3H2(g) 2NH3(g) Is 1.2 at this temperature, decide whether the system is at equilibrium. If it is not, predict which way the net reaction will proceed. The equilibrium constant (Kc) for the formation of nitrosyl chloride, and orange-yellow compound, from nitric oxide and molecular chlorine 2NO(g) + Cl2(g) 2NOCl is 6.5 x 104 at 35C. In a certain experiment, 2.0 x 10-2 mole of NO, 8.3 x 10-3 mole of Cl2. and 6.8 moles of NOCl are mixed in a 2.0-L flask. In which direction will the system proceed to reach equilibrium? Review of Concepts The equilibrium constant (Kc) for the A2 + B2 ↔ 2AB reaction is 3 at a certain temperature. Which of the diagrams shown here corresponds to the reaction at equilibrium? For those mixtures that are not at equilibrium, will the net reaction move in the forward or reverse direction to reach equilibrium? Calculating Equilibrium Concentrations 1. Express the equilibrium concentrations of all species in terms of the initial concentrations and a single unknown x, which represents the change in concentration. 2. Write the equilibrium constant expression in terms of the equilibrium concentrations. Knowing the value of the equilibrium constant, solve for x. 3. Having solved for x, calculate the equilibrium concentrations of all species. AP Chemistry Mr. Markic Page 8 of 12 Sample Exercise A mixture of 0.500 mol H2 and 0.500 mol I2 was placed in a 1.00-L stainless-steel flask at 430°C. The equilibrium constant Kc for the reaction H2(g) + I2(g) 2HI(g) is 54.3 at this temperature. Calculate the concentrations of H2, I2, and HI at equilibrium. For the same reaction and temperature as in the prior example, suppose that the initial concentrations of H2, I2, and HI are 0.00623 M, 0.00414 M, and 0.0224 M, respectively. Calculate the concentrations of these species at equilibrium Le Châtelier’s Principle If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position. Changes in Concentration N2 (g) + 3H2 (g) ↔ 2NH3 (g) Equilibrium shifts left to offset stress Add NH3 Change Increase concentration of product(s) Decrease concentration of product(s) Increase concentration of reactant(s) Decrease concentration of reactant(s) Shifts the Equilibrium left right right left AP Chemistry Mr. Markic Page 9 of 12 aA (g) + bB (g) cC (g) + dD (g) Change Increase concentration of product(s) Decrease concentration of product(s) Increase concentration of reactant(s) Decrease concentration of reactant(s) Shifts the Equilibrium Sample Exercise At 720°C, the equilibrium constant Kc for the reaction: N2(g) + 3H2(g) 2NH3(g) is 2.37 x 10-3. In a certain experiment the equilibrium concentrations are [N2] = 0.683 M, [H2] = 8.80 M, and [NH3] = 1.05 M. Suppose some NH3 is added to mixture so that its concentration is increased to 3.65 M (a) Use Le Chatelier’s principle to predict the shift in direction of the net reaction to reach a new equilibrium (b) Confirm your prediction by calculating the reaction quotient QC and comparing its value with Kc Practice Exercise At 430°C, the equilibrium constant (KP) for the reaction: 2NO(g) + O2(g) 2NO2(g) Is 1.5 x 105. In one experiment, the initial pressures of NO, O2, and NO2 are 2.1 x 10-3 atm, 1.1 x 10-2 atm, and 0.14 atm, respectively. Calculate QP, and predict the direction that the net reaction will shift to reach equilibrium Changes in Volume and Pressure A(g) + B(g) ↔ C(g) Change Increase pressure Decrease pressure Increase volume Decrease volume Shifts the Equilibrium Side with fewest moles of gas Side with most moles of gas Side with most moles of gas Side with fewest moles of gas AP Chemistry Mr. Markic Page 10 of 12 Consider the following equilibrium systems, predict the direction of the net reaction in each case as a result of increasing the pressure (decreasing the volume) on the system at constant temperature): (a) 2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g) (b) PCl5(g) PCl3(g) + Cl2(g) (c) H2(g) + CO2(g) H2O(g) + CO(g) Consider the equilibrium reaction involving nitrosyl chloride, nitric oxide, and molecular chlorine 2NOCl(g) ↔ 2NO(g) + Cl2(g) Predict the direction of the net reaction as a result of decreasing the pressure (increasing the volume) on the system at constant temperature. Review of Concepts The diagram here shows the gaseous reaction 2A ↔ A2 at equilibrium. If the pressure is decreased by increasing the volume at constant temperature, how would the concentrations of A and A2 change when a new equilibrium is established? Changes in Temperature Change Increase temperature Decrease temperature Exothermic Rx K decreases K increases Endothermic Rx K increases K decreases Adding a Catalyst • does not change K • does not shift the position of an equilibrium system • system will reach equilibrium sooner Catalyst lowers Ea for both forward and reverse reactions. Catalyst does not change equilibrium constant or shift equilibrium. uncatalyzed Le Châtelier’s Principle Change Concentration Shift Equilibrium yes Change Equilibrium Constant no Pressure yes* no Volume yes* no Temperature yes yes Catalyst no no *Dependent on relative moles of gaseous reactants and products catalyzed AP Chemistry Mr. Markic Page 11 of 12 Review of Concepts The diagram shown here represents the reaction X2 + Y2 ↔ 2XY at equilibrium at two temperatures (T2 > T1). Is the reaction endothermic or exothermic? Sample Exercise Consider the following equilibrium process between dinitrogen tetrafluoride (N2F4) and nitrogen difluoride (NF2): N2F4(g) 2NF2 (g) H° = 38.5 kJ/mol Predict the changes in the equilibrium if: (a) The reacting mixture is heated at constant volume (b) Some N2F4 gas is removed from the reacting mixture at constant temperature and volume (c) The pressure of the reacting mixture is decreased at constant temperature (d) A catalyst is added to the reacting mixture Practice Exercise Consider the equilibrium between molecular oxygen and ozone 3O2(g) ↔ 2O3(g) ΔH° = 284 kJ/mol What would be the effect of: (a) Increasing the pressure of the system by decreasing the volume (b) Adding O2 to the system at constant volume (c) Decreasing the temperature (d) Adding a catalyst AP Chemistry Mr. Markic Page 12 of 12 Big Idea 6: Any bond or intermolecular attraction that can be formed can be broken. These two processes are in dynamic competition, sensitive to initial conditions and external perturbations. Duration: Early March Textbook Chapter: 14 Enduring Understanding Essential Knowledge 6.A: Chemical equilibrium is a 6.A.1: In many classes of reactions, it is important to consider both the forward and dynamic, reversible state in which reverse reaction. rates of opposing processes are 6.A.2: The current state of a system undergoing a reversible reaction can be equal. characterized by the extent to which reactants have been converted to products. The relative quantities of reaction components are quantitatively described by the reaction quotient, Q. 6.A.3: When a system is at equilibrium, all macroscopic variables, such as concentrations, partial pressures, and temperature, do not change over time. Equilibrium results from an equality between the rates of the forward and reverse reactions, at which point Q = K. 6.A.4: The magnitude of the equilibrium constant, K, can be used to determine whether the equilibrium lies toward the reactant side or product side. 6.B: Systems at equilibrium are 6.B.1: Systems at equilibrium respond to disturbances by partially countering the effect responsive to external of the disturbance (Le Chatelier’s principle). perturbations, with the response 6.B.2: A disturbance to a system at equilibrium causes Q to differ from K, thereby taking leading to a change in the the system out of the original equilibrium state. The system responds by bringing Q back composition of the system. into agreement with K, thereby establishing a new equilibrium state. Learning Objective 5.16 The student can use Le Chatelier’s principle to make qualitative predictions for systems in which coupled reactions that share a common intermediate drive formation of a product. 5.17 The student can make quantitative predictions for systems involving coupled reactions that share a common intermediate, based on the equilibrium constant for the combined reaction. 5.18 The student can explain why a thermodynamically favored chemical reaction may not produce large amounts of product (based on consideration of both initial conditions and kinetic effects), or why a thermodynamically unfavored chemical reaction can produce large amounts of product for certain sets of initial conditions. 6.1 The student is able to, given a set of experimental observations regarding physical, chemical, biological, or environmental processes that are reversible, construct an explanation that connects the observations to the reversibility of the underlying chemical reactions or processes. 6.2 The student can, given a manipulation of a chemical reaction or set of reactions (e.g., reversal of reaction or addition of two reactions), determine the effects of that manipulation on Q or K. 6.3 The student can connect kinetics to equilibrium by using reasoning about equilibrium, such as Le Chatelier’s principle, to infer the relative rates of the forward and reverse reactions. 6.4 The student can, given a set of initial conditions (concentrations or partial pressures) and the equilibrium constant, K, use the tendency of Q to approach K to predict and justify the prediction as to whether the reaction will proceed toward products or reactants as equilibrium is approached. 6.5 The student can, given data (tabular, graphical, etc.) from which the state of a system at equilibrium can be obtained, calculate the equilibrium constant, K. 6.6 The student can, given a set of initial conditions (concentrations or partial pressures) and the equilibrium constant, K, use stoichiometric relationships and the law of mass action (Q equals K at equilibrium) to determine qualitatively and/or quantitatively the conditions at equilibrium for a system involving a single reversible reaction. 6.7 The student is able, for a reversible reaction that has a large or small K, to determine which chemical species will have very large versus very small concentrations at equilibrium. 6.8 The student is able to use Le Chatelier’s principle to predict the direction of the shift resulting from various possible stresses on a system at chemical equilibrium. 6.9 The student is able to use Le Chatelier’s principle to design a set of conditions that will optimize a desired outcome, such as product yield. 6.10 The student is able to connect Le Chatelier’s principle to the comparison of Q to K by explaining the effects of the stress on Q and K.









