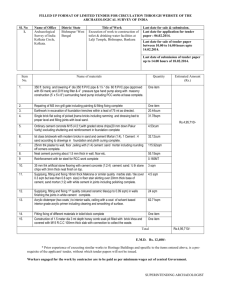
Application-of-Tarbela-Dam-Reservoir-TDR-silt-as-a-Pozzolan
advertisement

Application of Tarbela Dam Reservoir (TDR) silt as a Pozzolan and Production of Cement from TDR Silt Project Members Shah Rukh Khan (09PWCIV3203) Hasnain Khan (09PWCIV3209) Shoaib Muhammad (09PWCIV3286) Supervisor Dr. Mohammad Adil July 2013 Department of Civil Engineering, University of Engineering and Technology Peshawar Application of Tarbela Dam RESERVOIR (TDR) silt as a Pozzolan and Production of Cement from TDR Silt By Shah Rukh Khan (09PWCIV3203) Hasnain Khan (09PWCIV3209) Shoaib Muhammad (09PWCIV3286) A thesis presented to the University of Engineering and Technology, Peshawar in fulfillment of the thesis requirement for the degree of Bachelor of Science in Civil Engineering Peshawar, Khyber-Pakhtunkhwa, Pakistan, (2013) Certified by Accepted by ______________________ ___________________ Project Supervisor Chairman Dr. Mohammad Adil Civil Engineering Dept. ii AUTHOR'S DECLARATION I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. Shah Rukh Khan (09-PWCIV-3203) Hasnain Khan (09-PWCIV-3209) Shoaib Muhammad (09-PWCIV-3286) iii DEDICATION "We humbly thank Allah the Almighty, the Merciful and the Beneficent, who gave us health, thoughts and co-operative people to enable us achieve this goal." We dedicate this project to our supervisor: Dr. Mohammad Adil and our beloved Families and honorable teachers because they supported us and guided us at every difficult moment. May Allah bless them with everything he has ever created and may give us an opportunity to serve them the best way possible. (Ameen)! iv ACKNOWLEDGEMENT We would like to thank our supervisor Dr. Mohammad Adil for his guidelines and support. We also want to thank Engr. Anwar Shah (Tarbela Dam Project, WAPDA), Engr. Hazrat Umar (GM/PD, Tarbela Dam Project, WAPDA and his team members) and all those teachers in UET Peshawar for guiding us and providing information in their own fields, which really enabled us to accomplish our task with success. We are also very much thankful to Directorate of Science and Technology (DoST, KPK) for providing funding for this research. Also thanks to Engr. M. Waheed and Dr. Mehtab Faisal of PCSIR for their support and time they invested in this project. v ABSTRACT Deposition of silt in Tarbela Dam (TD) reservoir has become a major problem. Serious implications may result from silt deposition in the reservoir. Storage capacity of the reservoir has significantly been reduced and the chances of overflowing in critical situations like flooding have increased. Also due to low storing capacity the whole country is suffering from terrible power deficit throughout the year. Also the time to time, accidental, exclusion of silt through the penstocks and turbines cause serious damage to them. The idea of this project was to develop a sellable industrial product from this silt, out of several possible products listed by a past research project by Kashif and Idrees titled “Engineering Applications of Silt from TDR” we chose to do this project to sort out the application of silt as a pozzolan and as a major cement constituent. As dredging pumping or flushing silt from the reservoir is very costly or intensively environmentally hazardous, making this silt a revenue generating source will somewhat fulfill this cost and above all will become a productive source of sustainable improvement of economy by producing job opportunities. vi Table of Contents AUTHOR'S DECLARATION............................................................................................................... iii DEDICATION ....................................................................................................................................... iv ACKNOWLEDGEMENT ...................................................................................................................... v ABSTRACT........................................................................................................................................... vi List of Figures ......................................................................................................................................... x List of Tables ......................................................................................................................................... xi Chapter 1 ................................................................................................................................................. 1 Introduction ............................................................................................................................................. 1 1.1 Introduction ................................................................................................................................... 1 1.2 Background ................................................................................................................................... 1 1.2.1 Sedimentation Problem in Tarbela Dam (TD) Reservoir....................................................... 1 1.2.2 TD Reservoir Silt Status ........................................................................................................ 2 1.3 Silt ................................................................................................................................................. 4 1.3.1 Effect of Silt in Dam reservoir ............................................................................................... 5 1.4 Cement .......................................................................................................................................... 7 1.4.2 Constituents of Cement .......................................................................................................... 8 1.5 Pozzolan ........................................................................................................................................ 8 1.6 Cement Replacement .................................................................................................................... 9 1.5 Introduction to thesis................................................................................................................... 10 Chapter 2 ............................................................................................................................................... 12 Literature Review.................................................................................................................................. 12 2.1 Introduction:................................................................................................................................ 12 2.2Silt as Material: ............................................................................................................................ 12 2.2.1 General: ................................................................................................................................ 12 2.2.2 Silt Properties: ...................................................................................................................... 12 2.2.3 Uses of Silt: .......................................................................................................................... 14 2.3 Cement: ....................................................................................................................................... 17 2.3.1 History: ................................................................................................................................ 17 2.3.2Composition of Cement: ....................................................................................................... 18 2.3.3 Types of Cement: ................................................................................................................. 21 2.3.5 Standards .............................................................................................................................. 31 vii 2.4Pozzolan: ...................................................................................................................................... 31 2.5 Summary ..................................................................................................................................... 35 Chapter 3 ............................................................................................................................................... 36 Methodology ......................................................................................................................................... 36 3.1 Introduction ................................................................................................................................. 36 3.2 Tests on TD silt ........................................................................................................................... 36 3.3 Tests on off-the-shelf cement ...................................................................................................... 36 3.3.1 Fineness (ASTM C 184 – 94 Dry method for fineness) ...................................................... 36 3.3.2 Consistency (ASTM C 187) ................................................................................................. 36 3.3.3 Setting time (ASTM C 191 – 04) ......................................................................................... 36 3.3.4 Compressive strength (ASTM C 109/ C 109M) .................................................................. 37 3.4 Cement production from TDR silt .............................................................................................. 37 3.4.1 Different compositions for cement....................................................................................... 38 3.4.2 Clinkering ............................................................................................................................ 38 3.4.3 Grinding with Gypsum......................................................................................................... 38 3.4.4 Packaging ............................................................................................................................. 38 3.5 Tests on TD cement .................................................................................................................... 38 3.5.1 As pure cement .................................................................................................................... 38 3.5.2 As cement replacement ........................................................................................................ 38 3.6 Summary ..................................................................................................................................... 39 Chapter 4 ............................................................................................................................................... 40 Experimental work and Results ............................................................................................................ 40 4.1 Introduction ................................................................................................................................. 40 4.2 Analysis of TD silt ...................................................................................................................... 40 4.3 Tests on off-the shelf cement ...................................................................................................... 41 4.3.1 Fineness by dry process (ASTM C 184 - 94) ....................................................................... 41 4.3.2 Consistency (ASTM C 187) ................................................................................................. 42 4.3.3 Setting time (ASTM C 191 - 04) ......................................................................................... 42 4.3.4 Compressive strength (ASTM C 109/C 109M) ................................................................... 44 4.4 Production of TD cement in Laboratory ..................................................................................... 45 4.4 Tests on TD cement .................................................................................................................... 49 4.4.1 As pure cement .................................................................................................................... 49 4.4.2 As Cement replacement ....................................................................................................... 51 4.5 Summary ..................................................................................................................................... 54 Chapter 5 ............................................................................................................................................... 55 Discussion ............................................................................................................................................. 55 viii 5.1 Introduction ................................................................................................................................. 55 5.2 Analysis of Tarbela Dam Reservoir (TDR) silt .......................................................................... 55 5.3 Tests on off-the shelf cement ...................................................................................................... 55 5.4 Tests on TD Cement ................................................................................................................... 56 5.6 Summary ..................................................................................................................................... 59 Chapter 6 ............................................................................................................................................... 60 Conclusions ........................................................................................................................................... 60 6.1 Introduction ................................................................................................................................. 60 6.2 Conclusions ................................................................................................................................. 60 6.2.1 TD Cement Mortar mix........................................................................................................ 60 6.2.2 Low Energy Cement ............................................................................................................ 61 6.2.3 Storage capacity of TDR ...................................................................................................... 62 6.2.4 Revenue to accommodate heavy costs of dredging ............................................................. 62 6.2.5 Environmental protection..................................................................................................... 62 6.2.6 An idea of introducing cement plants in TD locality ........................................................... 62 6.2.7 Employment ......................................................................................................................... 62 6.2.7 Business competition ........................................................................................................... 63 6.3 Summary ..................................................................................................................................... 63 Chapter 7 ............................................................................................................................................... 64 Future plans........................................................................................................................................... 64 7.1 Introduction ................................................................................................................................. 64 7.2 Future recommendations ............................................................................................................. 64 7.2.1 Temperature ......................................................................................................................... 64 7.2.2 Silt sampling ........................................................................................................................ 64 7.2.3 Microscopic study of silt samples ........................................................................................ 64 7.2.4 Microscopic study of the cement produced ......................................................................... 65 7.2.5 Using sophisticated instruments........................................................................................... 65 7.2.6 Controlled environment ....................................................................................................... 65 7.2.7 Research for additives .......................................................................................................... 65 7.2.8 A special phenomenon ......................................................................................................... 65 7.2.9 Study the effects of alkalis present and their elimination .................................................... 66 7.3 Summary ..................................................................................................................................... 66 Appendix-A........................................................................................................................................... 67 Bibliography ......................................................................................................................................... 73 ix List of Figures Figure1.1: Comparative Sizes of soil particles ....................................................................................... 5 Figure1.2: Fly-Ash retention pond spill in Tennessee ............................................................................ 9 Figure1.3: Fly-Ash ................................................................................................................................ 10 Figure2.1: Production process of Cement ............................................................................................. 31 Figure3.1: Schematic diagram of TD Cement Production process ....................................................... 37 Figure3.2: Schematic diagram showing Project Overview ................................................................... 39 Figure4.1: Retained cement on Sieve #200 .......................................................................................... 42 Figure4.2: Vicat's Apparatus ................................................................................................................. 43 Figure4.3: Standard Sand ...................................................................................................................... 44 Figure4.4: Two moulds containing three cubes each ............................................................................ 45 Figure4.5: Hand mixing of TDR Silt and Chalk ................................................................................... 47 Figure4.6: Adding Molasses to the sample ........................................................................................... 47 Figure4.7: Pellets from the sample ....................................................................................................... 47 Figure4.8: Rotary kiln in laboratory ..................................................................................................... 48 Figure4.9: Clinkers produced................................................................................................................ 48 Figure4.10: Rod Mill Grinder in laboratory.......................................................................................... 49 Figure4.11: Broken cubes after Demoulding ........................................................................................ 50 Figure4.12: TD Cement mortar cubes from sample D .......................................................................... 51 Figure4.13: TD Cement mortar cubes from Sample A ......................................................................... 52 Figure4.14: TD Cement mortar cubes from Sample B ......................................................................... 53 Figure4.15: Mortar cubes made of sample C (marked as I) and sample D (marked as II) ................... 54 Figure5.1: TDR Silt Composition ......................................................................................................... 55 Figure5.2: Sample A composition ........................................................................................................ 57 Figure5.3: Sample B Composition ........................................................................................................ 57 Figure5.4: Sample C Composition ........................................................................................................ 58 Figure5.5: Sample D Composition........................................................................................................ 58 Figure6.1: TD Cement mortar mix Vs Type N and Type O mortar mix .............................................. 61 Figure6.2: 50% TD Cement +50% off-the Shelf Cement mortar mix Vs Type N and Type O mortar mix ........................................................................................................................................................ 61 x List of Tables Table1.1: The Size of Sand, Silt and Clay .............................................................................................. 4 Table1.2: Effects of Sedimentation in Reservoir .................................................................................... 5 Table2.1: Composition of Cement ........................................................................................................ 18 Table2.2: Usual Composition limits of Portland Cement ..................................................................... 20 Table2.3: General features of the main types of Portland Cement ....................................................... 24 Table4.1: Chemical Composition of TD silt ......................................................................................... 40 Table4.2: Properties of TD silt.............................................................................................................. 41 Table4.3: Initial setting time readings................................................................................................... 43 Table4.4: Different Compositions with TDR silt.................................................................................. 46 Table4.5: Compressive strength test readings of Sample D as pure cement......................................... 50 Table4.6: Compressive strength tests readings of sample A as cement replacement ........................... 51 Table4.7: Compressive strength tests readings of sample B ................................................................. 52 Table4.8: Compressive strength tests readings of Sample C as cement replacement ........................... 53 Table4.9: Compressive strength tests readings of Sample D ................................................................ 53 Table5.1: TD Cement Compressive strength as pure cement ............................................................... 59 Table5.2: TD Cement compressive strength as 50% off-the shelf cement replacement....................... 59 xi Chapter 1 Introduction 1.1 Introduction This chapter gives background of the project and introduction of two materials, the silt and the cement and their relation in context of TDR. Effect of silt in dam reservoir is discussed in detail. Also detail of sedimentation process in the reservoir is given in this chapter. Problem of sedimentation in Tarbela dam, its silt status and its impact on the reservoir are discussed in detail. The constituents of cement are also discussed. Pozzolan and their use as cement replacement are also enlightened. 1.2 Background Tarbela Dam on the Indus River in Pakistan is the second largest dam in the world by structural volume and the largest earth filled dam in the world. It is located in Haripur District, Khyber Pakhtunkhwa, about 50 kilometres (31 mi) northwest of Islamabad. The dam is 485 feet (148 m) high above the riverbed. The dam forms the Tarbela Reservoir, with a surface area of approximately 250-square-kilometre (97 sq. mi). The dam was completed in 1974 and was designed to store water from the Indus River for irrigation, flood control, and the generation of hydroelectric power. 1.2.1 Sedimentation Problem in Tarbela Dam (TD) Reservoir Because the source of the Indus River is glacial melt water from the Himalayas, the river carries huge amounts of sediment. The annual suspended sediment load is about 430 million tons. This means that, over time, the reservoir will fill. The useful life of the dam and reservoir was estimated to be somewhere around 50 years, since the dam's completion in 1976, meaning that the reservoir would have been full of sediment by 2030. However, sedimentation has been much lower than predicted, and it is now estimated that the useful lifespan of the dam will be 85 years, to about 2060. The River Indus carries a large volume of suspended sediments. It was analyzed on the basis of measurements carried out by the Irrigation Research Institute and Water and Power Development Authority (WAPDA) through a rating curve on discharge that the annual 1 suspended sediment load at Darband was 430 million ton per year or 0.26 million-acre feet (MAF) per year. It was assumed that the trap efficiency will be 100% until the gross capacity reduces to 4.5 MAF, thereafter, the trap efficiency will reduce to a uniform rate of 60%. The useful efficiency of Tarbela based on these assumptions was expected to be 50 years. It is natural to find silt and sediment in water but problems result when excess amounts are introduced into the water. Excess amounts can harmfully affect water quality, an essential component of fish habitat. Deposited silt and sediment can also be harmful to fish habitat. Some of the harmful impacts of silt and sediment deposits are: The clogging of the small spaces between gravel particles prevents the free flow of oxygenated water and the removal of waste products from developing eggs deposited in the gravel. This often suffocates the eggs and may make gravel beds unsuitable for egg incubation. The destruction of habitat for bottom dwelling organisms such as crayfish and insects. Fish rely on these organisms for food. The clogging of sheltered areas between boulders and gravel. Young fish need these areas as protection to survive. Since silt and sediment and the resulting turbidity can create a variety of harmful impacts to fish and fish habitat, it is important to avoid the introduction of these materials into the water. Planning and designing work projects with care and implementing environmentally friendly practices will protect fish and fish habitat. For most construction or development projects that cause the release of silt and sediment, there are effective methods for removing suspended sediment from the work site and preventing it from entering streams or lakes. Regardless of the above mentioned problems, the discharge carrying capacity of the reservoir is decreasing. In critical situations such as during flooding there may be a great chance of overflowing which may cause severe damage to human life and property. Also it may damage the operating machinery too. 1.2.2 TD Reservoir Silt Status Official sources in the WAPDA said that the World Bank had pledged US$3million for a feasibility study and the subsequent project. 2 According to official documents, the usable storage capacity at the time of Tarbela’s commissioning was 9.68 MAF, which has now reduced to 6.77 MAF due to sedimentation and is continuously on the decline. The feasibility study will be conducted by two multinational companies, Mott Macdonald Pvt Limited Pakistan and Hydraulics Research (HR) Walling Ford. They will determine the area of the lake to be cleansed and the total expenditure on the project. The companies will start work on the project in the second week of June this year as the letter of approval from General Manager TD has just been issued. The officials said the project will be completed in the next 15 months. The final report will be submitted to WAPDA and the World Bank. “Although it looks practically unfeasible to cleanse the thousand feet deep reservoir of silt, we hope to use advanced technology for this purpose as we are committed to increasing the life of the dam,” an official privy to the contract said. Sources revealed WAPDA had conducted five feasibility studies in the recent past. However, the plan was shelved due to unknown reasons. But the alarming increase in sedimentation had created a delta in the main dam close to the powerhouse, which requires immediate attention. That coupled with the power crisis got WAPDA thinking again about clearing sedimentation from the dam. The size of the delta, according to WAPDA’s recent annual report, was 50 miles long, 1 to 2 miles wide and 200 feet deep. The WAPDA authorities have attributed the sedimentation in the reservoir to unchecked deforestation in the catchment area of River Indus. The dam was built with a cost of $3 billion displacing a vast population of over 120 villages and small localities of district Haripur during 1968-1974. Initially built for irrigation purpose, TD also serves as the biggest source of hydel power generation in the country. The powerhouse consists of 14 power-generating units and was completed in three phases from 1977 to 1992-93 at a total cost of Rs16 billion. Its total generation capacity is 3,478 megawatts. Construction of another power house at tunnel No 4 is about to start next year. It would add 960 megawatts to the existing power generation in the national distribution system. 3 1.3 Silt Texture refers to the size of the particles that make up the soil. The terms sand, silt, and clay refer to relative sizes of the soil particles. Sand, being the larger size of particles, feels gritty. Silt, being moderate in size, has a smooth or floury texture. Clay, being the smaller size of particles, feels sticky. [Table 1.1 and Figure 1.1] Table1.1: The Size of Sand, Silt and Clay Name Particle Diameter Clay below 0.002 millimeters Silt 0.002 to 0.05 millimeters Very fine sand Fine sand Medium sand Coarse sand Very coarse sand 0.05 to 0.10 millimeters 0.10 to 0.25 millimeters 0.25 to 0.5 millimeters 0.5 to 1.0 millimeters 1.0 to 2.0 millimeters 2.0 to 75.0 millimeters Gravel Rock Greater than 75.0 millimeters(~2 in) Soil texture is classified according to particle size. Clay has the smallest particle and pore space size, followed by silt, and then by sand with the largest size particles. Soil texture is 4 very important for anticipating the potential infiltration, movement, and storage of soil water. Figure1.1: Comparative Sizes of soil particles 1.3.1 Effect of Silt in Dam reservoir All rivers contain sediments: a river, in effect, can be considered a body of flowing sediments as much as one of flowing water. When a river is stilled behind a dam, the sediments it contains sink to the bottom of the reservoir. The proportion of a river’s total sediment load captured by a dam – known as its "trap efficiency" – approaches 100 per cent for many projects, especially those with large reservoirs. As the sediments accumulate in the reservoir, so the dam gradually loses its ability to store water for the purposes for which it was built. Every reservoir loses storage to sedimentation although the rate at which this happens varies widely. Despite more than six decades of research, sedimentation is still probably the most serious technical problem faced by the dam industry shown in Table1.2. Table1.2: Effects of Sedimentation in Reservoir Cause Water level in downstream Watercourse Potential physical effects if the flow exceeds the channel capacity it will flood Potential environmental Effects Loss and damage to Property Land use affected property and land and damage bridges and bankside structures 5 Risk to human and animal life and health Cause Potential physical effects Rate of rise in water level in if too rapid it may wash away downstream watercourse animals and people scours river sediments Potential environmental Effects Risk to human and animal life and health Damage to river Ecology Appearance of river Affected erodes river banks Volume and velocity of Loss of land Risk to bankside property released water Damage to bankside Ecology washes out rooted aquatic Plants Appearance of river affected Loss of fish habitat and breeding sites Damage to river Ecology displaces fish downstream Fish removed from downstream river Damage to river Ecology Release of sediment from significant deposits in Damage to river Ecology reservoir downstream river if the load Loss of fish habitat and overwhelms the natural transport mechanism breeding sites Appearance of river affected 6 Cause Potential physical effects Potential environmental Effects if the reservoir sediment Deterioration in river contains pollutants these will quality, WFD objectives at be carried to downstream risk river Damage to river Ecology Long term habitat damage Effect on abstractions Release of poor quality from if the reservoir is stratified Release of fish from the river the reservoir released water will cause a reservoir deterioration of downstream Fish and other river quality e.g. low dissolved aquatic animals oxygen and elevated iron and killed manganese Effect on abstractions Deterioration in river quality, WFD objectives at risk Release of fish from the fish transferred from lake into reservoir downstream river Minor, some fish displaced from lake 1.4 Cement Cement, in the general sense of the word, can be described as a material with adhesive and cohesive properties which make it capable of bonding mineral fragments into a compact whole. This definition embraces a large variety of cementing materials. For constructional purposes, the meaning of the term ‘cement’ is restricted to the bonding materials used with stones, sand, bricks, building blocks, etc. The principal constituents of this type of cement are compounds of lime, so that in building and civil engineering we are concerned with calcareous cement. The cements of interest in the making of concrete have the property of setting and hardening under water by virtue of a chemical reaction with it and are, therefore, called hydraulic cements. Hydraulic cements consist mainly of silicates and aluminates of lime, and can be classified broadly as natural cements, Portland cements, and high-alumina cements. The present chapter 7 deals with the manufacture of Portland cement and its structure and properties, both when unhydrated and in a hardened state. 1.4.2 Constituents of Cement From the definition of Portland cement given above, it can be seen that it is made primarly from a calcareous material, such as limestone or chalk, and from alumina and silica found as clay or shale. Marl, a mixture of calcareous and argillaceous materials, is also used. Raw materials for the manufacture of Portland cement are found in nearly all countries and cement plants operate all over the world. The process of manufacture of cement consists essentially of grinding the raw materials, mixing them intimately in certain proportions and burning in a large rotary kiln at a temperature of up to about 1450 °C when the material sinters and partially fuses into balls known as clinker. The clinker is cooled and ground to a fine powder, with some gypsum added, and the resulting product is the commercial Portland cement so widely used throughout the world. 1.5 Pozzolan A pozzolan is a siliceous or siliceous and aluminous material which, in itself, possesses little or no cementitious value but which will, in finely divided form and in the presence of water, react chemically with calcium hydroxide at ordinary temperature to form compounds possessing cementitious properties (American Society for Testing and Materials (ASTM) C618). The broad definition of a pozzolan imparts no bearing on the origin of the material, only on its capability of reacting with calcium hydroxide and water. The general definition of a pozzolan embraces a large number of materials which vary widely in terms of origin, composition and properties. Both natural and artificial (man-made) materials show pozzolanic activity and are used as supplementary cementitious materials. Artificial pozzolans can be produced deliberately, for instance by thermal activation of kaolin-clays to obtain metakaolin, or can be obtained as waste or by-products from hightemperature process such as fly ashes from coal-fired electricity production. The most commonly used pozzolans today are industrial by-products such as fly-ash, silica fume from silicon smelting, highly reactive metakaolin, and burned organic matter residues rich in silica such as rice husk ash. Their use has been firmly established and regulated in many countries. However, the supply of high-quality pozzolanic by-products is limited and many local sources are already fully exploited. Alternatives to the established pozzolanic by-products are to be found on the one hand in an expansion of the range of industrial by-products or societal waste considered and on the other hand in an increased usage of naturally occurring pozzolans. Natural pozzolans are abundant in certain locations and are extensively used as an addition to Portland cement in countries such as for example Italy, Germany, Greece and China. The great majority of natural pozzolans in use today is of volcanic origin. Volcanic ashes and pumices largely composed of volcanic glass are commonly used, as are deposits in which the volcanic glass has been altered to zeolites by interaction with alkaline waters. Deposits of 8 sedimentary origin are less common. Diatomaceous earths, formed by the accumulation of siliceous diatom microskeletons, are a prominent source material here. 1.6 Cement Replacement A Pozzolanic material utilizes calcium hydroxide during its hydration phase to produce calcium silicate hydrate. Portland cement produced calcium hydroxide during hydration. Other pozzolanic materials such as fly-ash and silica fume can be used to replace a percentage of Portland cement in concrete used in industry. The use of these materials results in "Green Concrete". Such materials reduce solid waste (i.e. coal ash), require less water, and reduce carbon emissions (Naik and Moriconi 2005). Cement replacement would allow less Portland cement to be used in construction reducing carbon emissions from the manufacturing side of cement. Additionally substances used for replacement would be diverted from landfills. As a waste product, fly-ash for example can be toxic if it gets into the environment. In landfills, certain heavy metals can leach out of the ash into water supplies. Also, spills such as the fly-ash retention pond spill in Tennessee shown below would be reduced (Dewan 2008). Finally, replacement can add to the durability of structures and reduced expansion cracking (Naik and Moriconi 2005). Figure1.2: Fly-Ash retention pond spill in Tennessee Photo Courtesy of precast.org Fly-ash mixed with cement in the generation of concrete can provide greater strength over time than traditional industrial concrete. Fly-ash is a by-product from the burning of coal. The combustion process beginnings by pulverizing coal to a median diameter of 50 micron, or 50 micrometers. Once the coal is burned the resulting material is known as bottom ash and fly-ash. Bottom ash settles to the bottom of the furnace quickly due to its dense size. Fly-ash on the other hand settles slower since it is lighter. The fly-ash is easily separated in this manner. A cooling process then takes place that reduces the temperature of the fly-ash from 1500° C to 200° C. The cooling process is rapid and takes around 4 seconds to complete. The range of fly-ash particles 9 size is around 1-50 micron at the end of this process. The type of fly-ash produced depends on what type of coal was burned. The main classes of fly-ash are F and C. The two classes can be used in concrete as specified by ASTM. There are key chemical differences between the classes but the important fact is that both will improve the durability of concrete. This in turn adds to the sustainability and performance of the structure utilizing the concrete overtime (Helmuth 1987). Figure1.3: Fly-Ash Photo Courtesy of precast.org Fly-ash uses calcium hydroxide produced by Portland cement during hydration. Due to resulting chemical reactions, fly-ash itself will not hydrate until 15 days after a pour. The full hydration stage will not be reached until about 360 days. Yet the time delay is worth the benefit. The fly-ash Portland cement mix yields higher strength than just Portland cement mix. The graph shown in this slide represents this development over a 360 day period. In industry flyash replacement accounts for 20% of cement typically. There are research studies that display the benefits of increasing this percentage (Helmuth 1987). 1.5 Introduction to thesis In this chapter all the detailed information about TD Reservoir has been presented. The siltation and sedimentation processes from which TD Reservoir is suffering are explained in comprehensive manner. The negative impacts resulting from the said processes are explained in descriptive as well as tabulated form to comprehend easily. Texture of silt, Cement and its constituents has been briefly introduced. 10 Now-a-days in various parts of the world like India and Taiwan, they make use of reservoir silt in various industries like brick and tile industry, also in making concrete. The use of silt as raw material instead of clay or other material has been proved as cost effective. For this purpose the utilization of silt as an industrial resource in the form of both raw material for cement production and as pozzolan in cement replacement has been described in next chapters. 11 Chapter 2 Literature Review 2.1 Introduction In this chapter literature review about silt, cement and pozzolan is stated. Silt composition, its properties and the various uses of silt are discussed followed by cement history which includes origin of cement and the various forms in which it has been used up till now is also discussed. Composition of cement, various types of cement, the process by which cement is produced and various standards i.e. ASTM standards for cement production is also discussed. Finally pozzolans and their use in modern construction is discussed. 2.2Silt as Material 2.2.1 General Silt is granular material of a size somewhere between sand and clay whose mineral origin is quartz and feldspar. Silt may occur as a soil or as suspended sediment (also known as suspended load) in a surface water body. It may also exist as soil deposited at the bottom of a water body. It is solid, dust-like sediment that water, ice, and wind transport and deposit. Silt is created when rock is eroded, or worn away, by water and ice. As flowing water transports tiny rock fragments, they scrape against the sides and bottoms of stream beds, chipping away more rock. The particles grind against each other, becoming smaller and smaller until they are silt-size. Glaciers can also erode rock particles to create silt. Finally, wind can transport rock particles through a canyon or across a landscape, forcing the particles to grind against the canyon wall or one another. These particles are intermediate in size to sand and clay. The soil itself can be called silt if its silt content is greater than 80 percent. When deposits of silt are compressed and the grains are pressed together, rocks such as siltstone form. 2.2.2 Silt Properties Silt is made up of rock and mineral particles that are larger than clay but smaller than sand. Individual silt particles are so small that they are difficult to see. To be classified as silt, a particle must be less than.005 centimeters (.002 inches) across. Silty soil is slippery when wet, not grainy or rocky. The soil itself can be called silt if its silt content is greater than 80 percent. Because of adhering film of clay, they exhibit some plasticity, cohesions adhesion and absorption and can hold more amount of water than sand but less than clay. Soils dominated by silts armid way in properties, workability and productivity between sandy and clayey soils. Silt particles are silky or powdery to touch. They do not stick to one another like sand grains. It possesses low dry strength and its particles easily disintegrate in water..It is also soft and smooth, with individual pieces close together. It too holds a lot of water, but the slightly larger particles make it a little better at draining than clay. It is considered one of the better fertile soils. The average specific area of silt particles is 1 sp. m/g.Modulus of elasticity 12 is in the range of 2 to 20 N/mm2.Porosity is 35 to 50%,specific yield is on average 18 %.Hydraulic conductivity of silt is 106 to 104 cm/s. Engineering Properties of Silt: Silt—passes a No. 200 (0.075-mm or 75-μm) sieve. Soil is Nonplastic or very slightly plastic and that exhibits little or no strength when air-dry is silt. For classification, silt is a finegrained soil, or the fine-grained portion of a soil, with a plasticity index less than 4. Silts are different from clays in many important respects, but because of their similar appearance, they are often mistaken for each other, sometimes with unfortunate results. Dry, powdered silt and clay are indistinguishable, but they are easily identified by their behavior in the presence of water. Silts are the Nonplastic fines, they are inherently unstable in the presence of water and have a tendency to become "quick" when saturated that is, they assume the character of a viscous fluid and can flow. Silts are fairly impervious, difficult to compact, and highly susceptible to frost heaving. Silt masses undergo change of volume with change of shape (the property of dilatancy), in contrast with clays, which retain their volume with change of shape (the property of plasticity). The dilatancy of silt together with its quick reaction to vibration affords a means of identifying typical silt in the loose, wet state. When dry, silt can be pulverized easily under finger pressure (indicative of very slight dry strength), and has a smooth feel between the fingers unlike the grittiness of fine sand. Silts differ among themselves in size and shape of grains. This is reflected mainly in the property of compressibility. Generally, the higher the liquid limit of a silt, the more compressible it is. The liquid limit of a typical bulky-grained, inorganic silt is about 30 percent; whereas, highly micaceous or diatomaceous silts (elastic silts), consisting mainly of flaky grains, may have liquid limits as high as 100 percent. Some other engineering properties of silt are as follows; Little or no dry strength Nonplastic Volume change (settlement) under load is rapid Moderate to low permeability Susceptible to frost heave Minimal changes in volume due to wet/dry Very difficult to compact Very difficult to excavate below water table. 13 2.2.3 Uses of Silt In civil engineering it has the following uses It is used to build bricks Grow crops Make sedimentary rocks Make concrete Its industrial uses are discussed below: Dredged silt from the seabed of the Kochi port channels is seen as a viable alternative to clay used in the manufacture of tiles and bricks. In order to revive tile industry in Kerala, a host of marytime organizations has come up with a viable solution. The industry has been in dire straits for quite a few years, for want of clay, its main raw material. Scientific investigations have revealed that the massive quantity of the dredged silt from the seabed of Kochi port channels is a viable replacement of clay used in manufacture of tiles, bricks and similar products. On the other hand, China has been in the construction of rapid development period, a large number of new buildings rise abruptly out of ground at a cost of damage to farmland acres. Traditional clay brick production needs to dig field, it is contrary to the national policy of protecting cultivated land. National policy has expressly provided, the use of solid clay bricks in all cities was prohibited. And the use of river silt from dredging to produce clay wall materials was encouraged. In this way not only the cultivated land was saved but the dredged silt was also fully utilized and revenue was obtained. Its agriculture/product uses are: Silt dredged from a reservoir is not useless. It has a wide range of applications in the field of agriculture. It serves as a fertilizer and increases the fertility of soil. Some of the most common uses of silt as agriculture /product uses are as under; Aquaculture Construction Materials Decorative Landscaping Products Topsoil Aquaculture: Aquaculture of coastal fish, shellfish, and other species is a rapidly expanding worldwide industry. The expansion of aquaculture has led to a shortage of suitable sites in many areas, especially coastal sites. Lack of access, legal constraints, competing land uses, and high land costs have limited aquaculture development for many locations. One way these constraints may be overcome is to use maintenance dredged material containment areas for aquaculture Construction materials: 14 Some dredged material can be used as construction material. In some parts of the world, dredging to obtain construction material is a common practice. Since the demand for construction material is increasing day by day, in many cases, dredged material consists of a mixture of sand, silt and clay fractions, which requires some type of separation process. Dewatering may also be required because of high water content. Decorative landscaping products: Dredged material can be blended with recycled residual materials such as glass, gypsum, plastic bottles, and automobile interiors, etc. to manufacture statues, figures, garden benches, stepping patio pavers, plant vases, artificial rocks and water fountains. These products can be used to landscape gardens, backyards, swimming pool environments, monument stones, miniature golf courses, highway rest areas, tourist welcoming centers, zoos, and theme parks. Topsoil: Maintenance dredging in harbors, access channels, and rivers produces mixtures of sand, silt, clay and organic matter that can be excellent ingredients for topsoil. The dredged material may be used to improve soil structure for agricultural purposes. For production of food, uncontaminated material must be used. For other uses, the allowed contaminant level will depend on the use of the topsoil. In some cases, suitable material may be placed in a thin layer directly by pumping. After dewatering, the material is suitable topsoil for seeding and planting. Its environmental enhancement uses are: Fish & Wildlife Habitats Fisheries Improvement Wetland Restoration Uses related to fish and wildlife habitat are: Dredged material can be used beneficially to enhance or create various wildlife habitats. This may be either incidental to the project purpose or planned. For example, nesting meadows and habitat for large and small mammals and songbirds have been developed on upland or floodplain (seasonally flooded) dredged material placement sites. Numerous examples are available where dredged material has been used to create nesting islands for water birds and waterfowl. Many technical considerations are necessary for the creation of nesting islands. An island can be built where none existed, and vegetation states (bare ground versus sparse herb cover versus tree/shrub habitat) can be managed using periodic dredged material applications. The types of dredged material can be manipulated to provide proper substrates for nests; that is, softer silts and clays can be capped with sand, shell, and cobbles. The placement of the dredged material can be manipulated to provide the most acceptable habitat characteristics. 15 Upland wildlife habitats are typically dredged material containment areas that are no longer used or have long periods between maintenance dredged material placement. This allows native vegetation to grow and provide food and cover for wildlife. Site management is minimal, but can be intensified to provide special food crops, overwintering waterfowl feeding areas, and numerous other natural resource opportunities. Its uses related to fisheries improvement are: Appropriate placement of dredged material can improve ecological functions of fishery habitat. Fishery resource improvement can be demonstrated in several ways. Bottom relief created by mounding of dredged material may provide refuge habitat for fish. Fine-grained sediment transport can be stabilized by planting sea grasses or capping with shell or other coarse dredged material. The sea grasses or shell caps additionally improve fishery habitat. Uses related to wetland restoration are: Dredged material has been extensively used to restore and establish wetlands. Where proper sites can be located and government and private agency cooperation can be coordinated, wetlands restoration is a relatively common and technically feasible use of dredged material. Wetlands restoration or rehabilitation using dredged material is usually a more acceptable alternative to creation of a new wetland. Many of the world's natural wetlands are degraded or impacted, or have been destroyed, and the recovery of these wetlands is more important than creation of new ones. Most former wetlands still have hydric soils, even though the hydrologic characteristics of the site may have been altered. When a new wetland is created, hydric soil conditions, appropriate hydrologic conditions, and wetland vegetation must all be introduced to the site. Creation of a new wetland would also mean replacing one habitat type with another, which is not always desirable. Long-term planning, design, maintenance, and management are necessary to maintain a created wetland. Wetland restoration using dredged material can be accomplished in several ways. For example, dredged material can be applied in thin layers to bring degraded wetlands up to an intertidal elevation, as has been done extensively in south Louisiana. Dewatered dredged material can be used in wind and wave barriers to allow native vegetation to regrow and restore the viability of a wetland. Dredged material sediment can be used to stabilize eroding natural wetland shorelines or nourish subsiding wetlands. Dewatered dredged material can also be used to construct erosion barriers and other structures that aid in restoring a degraded or impacted wetland. Its uses related to construction industry are: In addition to using dredge material as construction fill as described above, sediment has been used as a raw material for manufacturing construction products such as building blocks, 16 tiles, and bricks. Technologies have been developed to produce these products, although fullscale commercial production is not yet available for all products. Building blocks have been manufactured from dredged material (both freshwater and marine). The proprietary process involves a blend of dredged material and industrial wastes such as ash or glass. Blocks have been used in construction projects such as noise barriers, security walls, and buildings. The manufacturing process involves high compressive forces to form the blocks rather than the heating process commonly used to produce bricks and blocks. Blocks formed in this process meet ASTM standards and can be used for a variety of building projects by unskilled labor. Production costs are estimated at between $20 and $80 per cubic yard to develop construction products from dredged material. 2.3 Cement: 2.3.1 History: The use of cementing materials is very old. The ancient Egyptians used calcined impure gypsum. The Greeks and the Romans used calcined limestone and later learned to add to lime and water, sand and crushed stone or brick and broken tiles. This was the first concrete in history. Lime mortar does not harden under water and for construction under water the Romans ground together lime and a volcanic ash or finely ground burnt clay tiles. The active silica and alumina in the ash and the tiles combined with the lime to produce what became known as pozzolanic cement from the name of the village of Pozzuoli, near Vesuvius, where the volcanic ash was first found. The name ‘pozzolanic cement’ is used to this day to describe cements obtained simply by the grinding of natural materials at normal temperature. Some of the Roman structures in which masonry was bonded by mortar, such as the Coliseum in Rome and the Pont du Gard near Nimes, and concrete structures such as the Pantheon in Rome, have survived to this day, with the cementitious material still hard and firm. In the ruins at Pompeii, the mortar is often less weathered than the rather soft stone. The middle Ages brought a general decline in the quality and use of cement, and it was only in the eighteenth century that an advance in the knowledge of cements occurred. John Smeaton, commissioned in 1756 to rebuild the Eddystone Lighthouse, off the Cornish coast, found that the best mortar was produced when pozzolana was mixed with limestone containing a considerable proportion of clayey matter. By recognizing the role of the clay, hitherto considered undesirable, Smeaton was the first to understand the chemical properties of hydraulic lime that is a material obtained by burning a mixture of lime and clay. There followed a development of other hydraulic cements, such as the ‘Roman cement’ obtained by James Parker by calcining nodules of argillaceous limestone, culminating in the patent for ‘Portland cement’ taken out by Joseph Aspdin, a Leeds bricklayer, stonemason, and builder, in 1824. This cement was prepared by heating a mixture of finely-divided clay and hard limestone in a furnace until CO2 had been driven off; this temperature was much lower than that necessary for clinkering. The prototype of modern cement was made in 1845 by 17 Isaac Johnson, who burnt a mixture of clay and chalk until clinkering, so that the reactions necessary for the formation of strongly cementitious compounds took place. The name ‘Portland cement’, given originally due to the resemblance of the colour and quality of the hardened cement to Portland stone – a limestone quarried in Dorset – has remained throughout the world to this day to describe a cement obtained by intimately mixing together calcareous and argillaceous, or other silica-, alumina-, and iron oxide-bearing materials, burning them at a clinkering temperature, and grinding the resulting clinker. The definition of Portland cement in various standards is on these lines, recognizing that gypsum is added after burning; nowadays, other materials may also be added or blended. 2.3.2Composition of Cement: The raw materials used in the manufacture of Portland cement consist mainly of lime, silica, alumina and iron oxide. These compounds interact with one another in the kiln to form a series of more complex products and, apart from a small residue of uncombined lime which has not had sufficient time to react, a state of chemical equilibrium is reached. However, equilibrium is not maintained during cooling, and the rate of cooling will affect the degree of crystallization and the amount of amorphous material present in the cooled clinker. The properties of this amorphous material, known as glass, differ considerably from those of crystalline compounds of a nominally similar chemical composition. Another complication arises from the interaction of the liquid part of the clinker with the crystalline compounds already present. Nevertheless, cement can be considered as being in frozen equilibrium, i.e. the cooled products are assumed to reproduce the equilibrium existing at the clinkering temperature. This assumption is, in fact, made in the calculation of the compound composition of commercial cements: the ‘potential’ composition is calculated from the measured quantities of oxides present in the clinker as if full crystallization of equilibrium products had taken place. Four compounds are usually regarded as the major constituents of cement: they are listed in Table 2.1 together with their abbreviated symbols. This shortened notation, used by cement chemists, describes each oxide by one letter, viz.: CaO = C; SiO2 = S; Al2O3 = A; and Fe2O3 = F. Likewise, H2O in hydrated cement is denoted by H, and SO3 by S Table2.1: Composition of Cement Name of compound Oxide composition Abbreviation Tricalcium silicate 3CaO.SiO2 C3S Dicalcium silicate 2CaO.SiO2 C2S Tricalcium aluminate 3CaO.Al2O3 C3A Tetracalcium aluminoferrite 4CaO.Al2O3.Fe2O3 C4AF 18 In reality, the silicates in cement are not pure compounds, but contain minor oxides in solid solution. These oxides have significant effects on the atomic arrangements, crystal form and hydraulic properties of the silicates. The calculation of the potential composition of Portland cement is based on the work of R. H. Bogue and others, and is often referred to as ‘Bogue composition’. Bogue’s equations for the percentages of main compounds in cement are given below. The terms in brackets represent the percentage of the given oxide in the total mass of cement. C3S = 4.07(CaO) – 7.60(SiO2) – 6.72(Al2O3) – 1.43(Fe2O3) – 2.85(SO3) C2S = 2.87(SiO2) – 0.75(3CaO.SiO2) C3A = 2.65(Al2O3) – 1.69(Fe2O3) C4AF = 3.04(Fe2O3). There are also other methods of calculating the composition. Bogue composition underestimates the C3S content (and overestimates C2S) because other oxides replace some of the CaO in C3S; as already stated, chemically pure C3S and C2S do not occur in Portland cement clinker. A modification of the Bogue composition which takes into account the presence of substituent ions in the nominally pure main compounds has been developed by Taylor for the rapidly cooled clinkers produced in modern cement plants. In addition to the main compounds listed in Table 2.1 there exist minor compounds, such as MgO, TiO2, Mn2O3, K2O and Na2O; they usually amount to not more than a few per cent of the mass of cement. Two of the minor compounds are of particular interest: the oxides of sodium and potassium, Na2O and K2O, known as the alkalis (although other alkalis also exist in cement). They have been found to react with some aggregates, the products of the reaction causing disintegration of the concrete, and have also been observed to affect the rate of the gain of strength of cement. It should, therefore, be pointed out that the term ‘minor compounds’ refers primarily to their quantity and not necessarily to their importance. The quantity of the alkalis and of Mn2O3 can be rapidly determined using a spectrophotometer. The compound composition of cement has been established largely on the basis of studies of phase equilibria of the ternary systems C–A–S and C–A–F, and the quaternary system C– C2S–C5A3–C4AF, and others. The course of melting or crystallization was traced and the compositions of liquid and solid phases at any temperature were computed. In addition to the methods of chemical analysis, the actual composition of clinker can be determined by a microscope examination of powder preparations and their identification by the measurement of the refractive index. Polished and etched sections can be used both in reflected and transmitted light. Other methods include the use of X-ray powder diffraction to identify the crystalline phases and also to study the crystal structure of some of the phases, and of differential thermal analysis; quantitative analysis is also possible, but complicated calibrations are involved. Modern techniques include phase analysis through a scanning 19 electron microscope and image analysis through an optical microscope or a scanning electron microscope. Estimating the composition of cement is aided by more rapid methods of determining the elemental composition, such as X-ray fluorescence, X-ray spectrometry, atomic absorption, flame photometry, and electron probe micro-analysis. X-ray diffractometry is useful in the determination of free lime, i.e. CaO as distinct from Ca(OH)2, and this is convenient in controlling the kiln performance. C3S, which is normally present in the largest amounts, occurs as small, equidimensional colourless grains. On cooling below 1250 °C, it decomposes slowly but, if cooling is not too slow, C3S remains unchanged and is relatively stable at ordinary temperatures. C2S is known to have three, or possibly even four, forms. a-C2S, which exists at high temperatures, inverts to the ß-form at about 1450 °C. ß-C2S undergoes further inversion to?C2S at about 670 °C but, at the rates of cooling of commercial cements, ß-C2S is preserved in the clinker. ß-C2S forms rounded grains, usually showing twinning. C3A forms rectangular crystals, but C3A in frozen glass forms an amorphous interstitial phase. C4AF is really a solid solution ranging from C2F to C6A2F, but the description C4AF is a convenient simplification. The actual proportions of the various compounds vary considerably from cement to cement, and indeed different types of cement are obtained by suitable proportioning of the raw materials. In the United States, an attempt was at one time made to control the properties of cements required for different purposes by specifying the limits of the four major compounds, as calculated from the oxide analysis. This procedure would cut out numerous physical tests normally performed, but unfortunately the calculated compound composition is not sufficiently accurate, nor does it take into account all the relevant properties of cement, and cannot therefore serve as a substitute for direct testing of the required properties. A general idea of the composition of cement can be obtained from Table 2.2, which gives the oxide composition limits of Portland cements. Table2.2: Usual Composition limits of Portland Cement Oxide CaO SiO2 Al2O3 Fe2O3 MgO Alkalis (as Na2O) SO3 20 Content,% 60-67 17-25 3-8 0.5-6.0 0.5-4.0 0.3-1.2 2.0-3.5 Functions of Various Ingredients of Cement: 1. Lime: It is very important in cement. It forms about 63 percent of cement. The nature of cement depends on it. Deficiency in lime reduces the strength of cement and causes it to set quickly. Again excess cause cement unsound and cause disintegration and expansion. 2. Silica: It is another important compound. It imparts strength to concrete. 3. Alumina: It imparts quick setting property of cement. Clinkering temperature is lowered by adding of alumina. 4. Magnesia: It should not present in cement more than 2 percent. Excess reduce strength. 5. Iron oxide: It imparts the colour of cement. At high temperature it goes to a chemical reaction with calcium and aluminum and forms Tetracalcium aluminoferrite which imparts hardness and strength. 6. Sulphur tri oxide: It should not present more than 2 percent. Excess causes cement to be unsound. 7. Calcium sulphate: It is present in cement in the form of gypsum. It retards the setting of cement. 8. Alkalies: It should not present more than 1 percent. Excess causes efflorescence. 2.3.3 Types of Cement: Originally, concrete was made using a mixture of only three materials: cement, aggregate, and water; almost invariably, the cement was Portland cement. Later on, in order to improve some of the properties of concrete, either in the fresh or in the hardened state, very small quantities of chemical products were added into the mix. These chemical admixtures, often called simply admixtures. Later still, other materials, inorganic in nature, were introduced into the concrete mix. The original reasons for using these materials were usually economic: they were cheaper than Portland cement, sometimes because they existed as natural deposits requiring no, or little, processing, sometimes because they were a byproduct or waste from industrial processes. For practical purposes of selection of an appropriate Portland cement or blended cement, it is useful to consider a classification based on the relevant physical or chemical property, such as a rapid gain of strength, low rate of evolution of the heat of hydration, or resistance to sulphate attack. A list of different Portland cements is given below. Ordinary Portland cement: This is by far the most common cement in use: about 90 per cent of all cement used in the United States (total production in 2008 of about 73 million tonnes per annum) and a like percentage of the ordinary type in the United Kingdom (total production of 12 million tonnes 21 per annum in 2005). It may be interesting to note that in 2007 the annual consumption of cement in the United Kingdom was equivalent to nearly 250 kg per head of population: the corresponding figure for the United States was 360 kg. For every man, woman and child in the world, the consumption in 2007 was 420 kg per annum, which is second only to the consumption of water. The biggest change occurred in China, where the increase between 1995 and 2004 was 90%. Rapid-hardening Portland cement: This cement comprises Portland cement subclasses of 32.5 and 42.5 MPa as prescribed by BS EN 197-1: 2000. Rapid-hardening Portland cement (Type III), as its name implies, develops strength more rapidly, and should, therefore, be correctly described as high early strength cement. The rate of hardening must not be confused with the rate of setting: in fact, ordinary and rapid-hardening cements have similar setting times, prescribed by BS 12: 1996 as an initial setting time of not less than 45 minutes. The final setting time is no longer prescribed. BS EN 197-1: 2000 does not prescribe fineness. The increased rate of gain of strength of the rapid-hardening Portland cement is achieved by a higher C3S content (higher than 55 per cent, but sometimes as high as 70 per cent) and by a finer grinding of the cement clinker. British Standard BS 12: 1996, unlike previous versions of BS 12, does not prescribe the fineness of cement, either ordinary or rapidhardening. However, the standard provides for an optional controlled fineness Portland cement and so does BS EN 197-1: 2000. The range of fineness is agreed between the manufacturer and the user. Such cement is valuable in applications where it makes it easier to remove excess water from the concrete during compaction because the fineness is more critical than the compressive strength. Special Very Rapid-hardening Portland Cements: There exist several specially manufactured cements which are particularly rapid-hardening. One of these, a so-called ultra high early strength cement. This type of cement is not standardized but rather supplied by individual cement manufacturers. Generally, the rapid strength development is achieved by grinding the cement to a very high fineness: 700 to 900 m2/kg. Because of this, the gypsum content has to be higher (4 per cent expressed as SO3) than in cements complying with BS EN 197-1 : 2000, but in all other respects the ultra high early strength cement satisfies the requirements of that standard. It can be noted that the high gypsum content has no adverse effect on long-term soundness as the gypsum is used up in the early reactions of hydration. Low Heat Portland cement: The rise in temperature in the interior of a large concrete mass due to the heat development by the hydration of cement, coupled with a low thermal conductivity of concrete, can lead to serious cracking. For this reason, it is necessary to limit the rate of heat evolution of the cement used in this type of structure: a greater proportion of the heat can then be dissipated and a lower rise in temperature results. 22 Cement having such a low rate of heat development was first produced for use in large gravity dams in the United States, and is known as low heat Portland cement (Type IV). However, for some time now, Type IV cement has not been produced in the United States. In the United Kingdom, low heat Portland cement is covered by BS 1370 : 1979, which limits the heat of hydration of this cement to 250 J/g (60 cal/g) at the age of 7 days, and 290 J/g (70 cal/g) at 28 days. Sulphate-resisting Cement: In discussing the reactions of hydration of cement, and in particular the setting process, mention was made of the reaction between C3A and gypsum (CaSO4.2H2O) and of the consequent formation of calcium sulfoaluminate. In hardened cement, calcium aluminate hydrate can react with a sulphate salt from outside the concrete in a similar manner: the product of addition is calcium sulfoaluminate, forming within the framework of the hydrated cement paste. Because the increase in the volume of the solid phase is 227 percent, gradual disintegration of concrete results. A second type of reaction is that of Base Exchange between calcium hydroxide and the sulphates, resulting in the formation of gypsum with an increase in the volume of the solid phase of 124 per cent. White Cement and Pigments: For architectural purposes, white or a pastel colour concrete is sometimes required. To achieve best results it is advisable to use white cement with, of course, a suitable fine aggregate and, if the surface is to be treated, also an appropriate coarse aggregate. White cement has also the advantage that it is not liable to cause staining because it has a low content of soluble alkalis. White Portland cement is made from raw materials containing very little iron oxide (less than 0.3 per cent by mass of clinker) and manganese oxide. China clay is generally used, together with chalk or limestone, free from specified impurities. Oil or gas is used as fuel for the kiln in order to avoid contamination by coal ash. Since iron acts as a flux in clinkering, its absence necessitates higher kiln temperatures (up to 1650 °C) but sometimes cryolite (sodium aluminum fluoride) is added as a flux. Portland Blastfurnace Cement: Cements of this name consist of an intimate mixture of Portland cement and ground granulated blastfurnace slag (in ASTM parlance, simply slag). This slag is a waste product in the manufacture of pig iron, about 300 kg of slag being produced for each tonne of pig iron. Chemically, slag is a mixture of lime, silica, and alumina, that is, the same oxides that make up Portland cement but not in the same proportions. There exist also non-ferrous slags; their use in concrete may become developed in the future. Blastfurnace slag varies greatly in composition and physical structure depending on the processes used and on the method of cooling of the slag. For use in the manufacture of blastfurnace cement, the slag has to be quenched so that it solidifies as glass, crystallization 23 being largely prevented. This rapid cooling by water results also in fragmentation of the material into a granulated form. Pelletizing, which requires less water, can also be used. Supersulphated Cement: Supersulphated cement is made by intergrinding a mixture of 80 to 85 per cent of granulated blastfurnace slag with 10 to 15 per cent of calcium sulfate (in the form of dead-burnt gypsum or anhydrite) and up to 5 per cent of Portland cement clinker. A fineness of 400 to 500 m2/kg is usual. Supersulphated cement is thus fundamentally different from Portland cement, in which calcium silicate is the main component. The cement has to be stored under very dry conditions as otherwise it deteriorates rapidly. Supersulphated cement is used extensively in Belgium, where it is known as ciment métallurgique sursulfaté, also in France, and was previously manufactured in Germany (under the name of Sulfathüttenzement). In the United Kingdom, the cement was covered by BS 4248- : 2004 (withdrawn) but, because of production difficulties, the manufacture of the cement has been discontinued. The European standard for supersulphated cement is BS EN 15743: 2010, which gives physical and chemical requirements. The ASTM has designated five types of Portland cement, designated Types I-V: Table2.3: General features of the main types of Portland cement Classification Type I General purpose Type II Moderate sulphate resistance Type III High early strength Low heat of hydration (slow reacting) Characteristics Applications Fairly high C3S content for good General construction (most early strength development buildings, bridges, pavements, precast units, etc) Low C3A content (<8%) Structures exposed to soil or water containing sulphate ions Ground more finely, may have Rapid construction, cold slightly more C3S weather concreting Low content of C3S (<50%) and Massive structures such as C3A dams. Now rare Type IV Type V High sulphate resistance Very low C3A content (<5%) White White colour No C4AF, low MgO 24 Structures exposed to high levels of sulphate ions Decorative (otherwise has properties similar to Type I) In Addition To Ordinary Portland cement there are Many Varieties of Cement. Important Varieties Are Briefly Explained Below: (i) White Cement: The cement when made free from colouring oxides of iron, manganese and chlorium results into white cement. In the manufacture of this cement, the oil fuel is used instead of coal for burning. White cement is used for the floor finishes, plastering, ornamental works etc. In swimming pools white cement is used to replace glazed tiles. It is used for fixing marbles and glazed tiles. (ii) Coloured Cement: The cements of desired colours are produced by intimately mixing pigments with ordinary cement. The chlorium oxide gives green colour. Cobalt produce blue colour. Iron oxide with different proportion produce brown, red or yellow colour. Addition of manganese dioxide gives black or brown coloured cement. These cements are used for giving finishing touches to floors, walls, window sills, roofs etc. (iii) Quick Setting Cement: Quick setting cement is produced by reducing the percentage of gypsum and adding a small amount of aluminum sulphate during the manufacture of cement. Finer grinding also adds to quick setting property. This cement starts setting within 5 minutes after adding water and becomes hard mass within 30 minutes. This cement is used to lay concrete under static or slowly running water. (iv) Rapid-Hardening Cement: This cement can be produced by increasing lime content and burning at high temperature while manufacturing cement. Grinding to very fine is also necessary. Though the initial and final setting time of this cement is the same as that of Portland cement, it gains strength in early days. This property helps in earlier removal of form works and speed in construction activity. (v) Low Heat Cement: In mass concrete works like construction of dams, heat produced due to hydration of cement will not get dispersed easily. This may give rise to cracks. Hence in such constructions it is preferable to use low heat cement. This cement contains low percentage (5%) of Tricalcium aluminate (C3A) and higher percentage (46%) of Dicalcium silicate (C2S). (vi) Pozzulana Cement Pozzulana is a volcanic powder found in Italy. It can be processed from shale and certain types of clay also. In this cement pozzulana material is 10 to 30 per cent. It can resist action of sulphate. It releases less heat during setting. It imparts higher degree of water 25 tightness. Its tensile strength is high but compressive strength is low. It is used for mass concrete works. It is also used in sewage line works. (vii) Expanding Cement: This cement expands as it sets. This property is achieved by adding expanding medium like sulpho aluminate and a stabilizing agent to ordinary cement. This is used for filling the cracks in concrete structures. (viii) High Alumina Cement: It is manufactured by calcining a mixture of lime and bauxite. It is more resistant to sulphate and acid attack. It develops almost full strength within 24 hours of adding water. It is used for under water works. (ix) Blast Furnace Cement: In the manufacture of pig iron, slag comes out as a waste product. By grinding clinkers of cement with about 60 to 65 per cent of slag, this cement is produced. The properties of this cement are more or less same as ordinary cement, but it is cheap, since it utilise waste product. This cement is durable but it gains the strength slowly and hence needs longer period of curing. (x) Acid Resistant Cement: This cement is produced by adding acid resistant aggregated such as quartz, quartzite, sodium silicate or soluble glass. This cement has good resistance to action of acid and water. It is commonly used in the construction of chemical factories. (xi) Sulphate Resistant Cement: By keeping the percentage of Tricalcium aluminate C3A below five per cent in ordinary cement this cement is produced. It is used in the construction of structures which are likely to be damaged by alkaline conditions. Examples of such structures are canals, culverts etc. (xii) Fly Ash Blended Cement: Fly ash is a byproduct in thermal stations. The particles of fly ash are very minute and they fly in the air, creating air pollution problems. Thermal power stations have to spend lot of money to arrest fly ash and dispose safely. It is found that one of the best way to dispose fly ash is to mix it with cement in controlled condition and derive some of the beneficiary effects on cement. Nowadays cement factories produce the fly ash in their own thermal stations or borrow it from other thermal stations and further process it to make it suitable to blend with cement. 20 to 30% fly ash is used for blending. Fly ash blended cements have superior quality of resistance to weathering action. The ultimate strength gained is the same as that with ordinary Portland cement. However strength gained in the initial stage is slow 26 (xiii)Oil-well Cement: Oil-well cement is a specially designed variety of hydraulic cement produced with gray Portland clinker. It usually forges slowly and is manageable at high temperatures and pressures. Produced in classes from A to H and J, oil-well cement is applicable for different depth, chemical aggression, or pressure levels. (xiv) Blended Cement: Blended hydraulic cements are produced by intergrinding or blending Portland cement and supplementary cementitious materials or SCM such as ground granulated blast furnace slag, fly ash, silica fume, calcined clay, hydrated lime, and other pozzolans. The use of blended cements in ready-mix concrete reduces mixing water and bleeding, improves workability and finishing, inhibits sulphate attack and the alkali-aggregate reaction, and reduces the heat of hydration. 2.3.4 Cement Production From the definition of Portland cement it is clear that it is made primarly from a calcareous material, such as limestone or chalk, and from alumina and silica found as clay or shale. Marl, a mixture of calcareous and argillaceous materials, is also used. Raw materials for the manufacture of Portland cement are found in nearly all countries and cement plants operate all over the world. The process of manufacture of cement consists essentially of grinding the raw materials, mixing them intimately in certain proportions and burning in a large rotary kiln at a temperature of up to about 1450 °C when the material sinters and partially fuses into balls known as clinker. The clinker is cooled and ground to a fine powder, with some gypsum added, and the resulting product is the commercial Portland cement so widely used throughout the world. The mixing and grinding of the raw materials can be done either in water or in a dry condition; hence the names ‘wet’ and ‘dry’ processes. The actual methods of manufacture depend also on the hardness of the raw materials used and on their moisture content. Considering first the wet process. When chalk is used, it is finely broken up and dispersed in water in a washmill; this is a circular pit with revolving radial arms carrying rakes which break up the lumps of solid matter. The clay is also broken up and mixed with water, usually in a similar washmill. The two mixtures are now pumped so as to mix in predetermined proportions and pass through a series of screens. The resulting cement slurry flows into storage tanks. When limestone is used, it has to be blasted, then crushed, usually in two progressively smaller crushers, and then fed into a ball mill with the clay dispersed in water. There, the comminution of the limestone (to the fineness of flour) is completed, and the resultant slurry is pumped into storage tanks. From here onwards, the process is the same regardless of the original nature of the raw materials. 27 The slurry is a liquid of creamy consistency, with a water content of between 35 and 50 per cent, and only a small fraction of material – about 2 per cent – larger than a 90 µm (No. 170 ASTM) sieve size. There are usually a number of storage tanks in which the slurry is kept, the sedimentation of the suspended solids being prevented by mechanical stirrers or bubbling by compressed air. The lime content of the slurry is governed by the proportioning of the original calcareous and argillaceous materials, as mentioned earlier. Final adjustment in order to achieve the required chemical composition can be made by blending slurries from different storage tanks, sometimes using an elaborate system of blending tanks. Occasionally, for example in the world’s northernmost plant in Norway, the raw material is a rock of such composition that it alone is crushed and no blending is required. Finally, the slurry with the desired lime content passes into the rotary kiln. This is a large, refractory-lined steel cylinder, up to 8 m (or 26 ft) in diameter, sometimes as long as 230 m (or 760 ft), slowly rotating about its axis, which is slightly inclined to the horizontal. The slurry is fed in at the upper end while pulverized coal is blown in by an air blast at the lower end of the kiln, where the temperature reaches about 1450 °C. The coal, which must not have too high an ash content, deserves a special mention because typically 220 kg (500 lb) of coal is used to make one tonne of cement. This is worth bearing in mind when considering the price of cement. Oil (of the order of 125 litres (33 US gallons) per tonne of cement) or natural gas were also used, but since the 1980s most oil-fired plants have been converted to coal, which is by far the most common fuel used in most countries. It is worth noting that, because it is burnt in the kiln, coal with a high sulfur content can be used without harmful emissions. The slurry, in its movement down the kiln, encounters a progressively higher temperature. At first, the water is driven off and CO2 is liberated; further on, the dry material undergoes a series of chemical reactions until finally, in the hottest part of the kiln, some 20 to 30 per cent of the material becomes liquid, and lime, silica and alumina recombine. The mass then fuses into balls, 3 to 25 mm in diameter, known as clinker. The clinker drops into coolers, which are of various types and often provide means for an exchange of heat with the air subsequently used for the combustion of the pulverized coal. The kiln has to operate continuously in order to ensure a steady regime, and therefore uniformity of clinker, and also to reduce the deterioration of the refractory lining. It should be noted that the flame temperature reaches 1650 °C. The largest existing kiln in a wet process plant produces 3600 tonnes of clinker a day. Because the manufacture of cement by the wet process is energy intensive, new wet process plants are no longer built. In the dry and semi-dry processes, the raw materials are crushed and fed in the correct proportions into a grinding mill, where they are dried and reduced in size to a fine powder. The dry powder, called raw meal, is then pumped to a blending silo, and final adjustment is now made in the proportions of the materials required for the manufacture of cement. To obtain a uniform and intimate mixture, the raw meal is blended, usually by means of compressed air inducing an upward movement of the powder and decreasing its apparent density. The air is pumped over one quadrant of the silo at a time, and this permits the apparently heavier material from the non-aerated quadrants to move laterally into the aerated quadrant. Thus the aerated material tends to behave almost like a liquid and, by aerating all 28 quadrants in turn for a total period of about one hour, a uniform mixture is obtained. In some cement plants, continuous blending is used. In the semi-dry process, the blended meal is now sieved and fed into a rotating dish called a granulator, water weighing about 12 per cent of the meal being added at the same time. In this manner, hard pellets about 15 mm in diameter are formed. This is necessary, as cold powder fed direct into a kiln would not permit the air flow and exchange of heat necessary for the chemical reactions of formation of cement clinker. The pellets are baked hard in a pre-heating grate by means of hot gases from the kiln. The pellets then enter the kiln, and subsequent operations are the same as in the wet process of manufacture. Since, however, the moisture content of the pellets is only 12 per cent as compared with the 40 per cent moisture content of the slurry used in the wet process, the semi-dry process kiln is considerably smaller. The amount of heat required is also very much lower because only some 12 per cent of moisture has to be driven off, but additional heat has already been used in removing the original moisture content of the raw materials (usually 6 to 10 per cent). The processes are thus quite economical, but only when the raw materials are comparatively dry. In such a case the total coal consumption can be as little as 100 kg (220 lb) per tonne of cement. In the dry process, the raw meal, which has a moisture content of about 0.2 per cent, is passed through a pre-heater, usually of a suspension type; that means that the raw meal particles are suspended in the rising gases. Here, the raw meal is heated to about 800 °C before being fed into the kiln. Because the raw meal contains no moisture to be driven off and because it is already pre-heated, the kiln can be shorter than in the wet process. The preheating uses the hot gas leaving the kiln. Because that gas contains a significant proportion of the rather volatile alkalis and chlorides, a part of the gas may need to be bled off to ensure that the alkali content of the cement is not too high. The major part of the raw meal can be passed through a fluidized calciner (using a separate heat source) introduced between the pre-heater and the kiln. The temperature in the fluidized calciner is about 820 °C. This temperature is stable so that the calcination is uniform and the efficiency of the heat exchange is high. A part of the raw meal is fed direct into the kiln in the usual manner but, overall, the effect of the fluidized calciner is to increase the decarbonation (dissociation of CaCO3) of the raw meal prior to entry into the kiln and thus greatly to increase the kiln throughput. What is probably the largest dry process plant in the world produces 10 000 tonnes of clinker a day using a kiln 6.2 m (20 ft) in diameter and 105 m (345 ft) long. In the U.S. more than 80% of cement production uses the dry process. It should be stressed that all processes require an intimate mixture of the raw materials because a part of the reactions in the kiln must take place by diffusion in solid materials, and a uniform distribution of materials is essential to ensure a uniform product. 29 On exit from the kiln, regardless of the type of process, the clinker is cooled, the heat being used to preheat the combustion air. The cool clinker, which is characteristically black, glistening, and hard, is interground with gypsum in order to prevent flash setting of the cement. The grinding is done in a ball mill consisting of several compartments with progressively smaller steel balls, sometimes preceded by passing through a roll press. In most plants, a closed-circuit grinding system is used: the cement discharged by the mill is passed through a separator, fine particles being removed to the storage silo by an air current, while the coarser particles are passed through the mill once again. Closed-circuit grinding avoids the production of a large amount of excessively fine material or of a small amount of too coarse material, faults often encountered with open-circuit grinding. Small quantities of grinding aids such as ethylene glycol or propylene glycol are used. Information about grinding aids is given by Massazza and Testolin.The performance of a ball mill can be improved by pre-grinding the clinker in a horizontal impact crusher. Once the cement has been satisfactorily ground, when it will have as many as 1.1 × 1012 particles per kg (5 × 1011 per lb), it is ready for transport in bulk. Less commonly, the cement is packed in bags or drums. However, some types of cement, such as white, hydrophobic, expansive, regulated-set, oil-well, and high alumina, are always packed in bags or drums. A standard bag in the United Kingdom contains 50 kg (110 lb) of cement; a US sack weighs 94 lb (42.6 kg); other bag sizes are also used. Bags of 25 kg are becoming popular. Except when the raw materials necessitate the use of the wet process, the dry process is used nowadays in order to minimize the energy required for burning. Typically, the burning process represents 40 to 60 per cent of the production cost, while the extraction of raw materials for the manufacture of cement represents only 10 per cent of the total cost of cement. Around 1990, the average energy consumption in the United States for the production of 1 tonne of cement by the dry process was 1.6 MWh. In modern plants, this figure is much reduced, being below 0.8 MWh in Austria. Electricity consumption, which accounts for some 6 to 8 per cent of total energy used, is typically of the order: 10 kWh for crushing the raw materials, 28 kWh in the raw meal preparation, 24 kWh in burning, and 41 kWh in grinding. The capital cost of installation of a cement plant is very high: nearly US$200 per tonne of cement produced per annum. In addition to the main processes, there are also other processes of manufacture of cement, of which one, using gypsum instead of lime, perhaps deserves mention. Gypsum, clay and coke with sand and iron oxide are burnt in a rotary kiln, the end products being Portland cement and sulfur dioxide which is further converted into sulfuric acid. In areas where only a small cement production is required or where investment capital is limited, a vertical kiln of the Gottlieb type can be used. This fires nodules of raw meal and fine coal powder combined, and produces agglomerated clinker which is then broken up. A single kiln, 10 m (33 ft) high, produces up to 300 tonnes of cement a day. China used several 30 thousand of such kilns, but now has a very large modern cement industry, producing 1000 million tonnes per annum. Figure2.1: Production process of Cement 2.3.5 Standards ASTM (American society of testing materials) cement and concrete standards are instrumental in the evaluation and testing of concrete, cement, and aggregates. Concrete can have different properties depending upon the mixture that is used in creating it, which contains cement, chemical admixtures, and aggregates. These ingredients are mixed with water to create concrete which is used as a primary construction material in buildings. These cement and concrete standards allow laboratories all over the world to test and evaluate concrete mixtures to ensure their strength and safety. These standards help to identify the various properties of concrete including strength, elasticity, hardness, and workability. For ASTM's cement and concrete standards refer to Appendix-A. 2.4 Pozzolan Pozzolan is a natural or artificial material containing silica in a reactive form. A more formal definition of ASTM 618-08a describes pozzolan as a siliceous or siliceous and aluminous material which in itself possesses little or no cementitious value but will, in finely-divided 31 form and in the presence of moisture, chemically react with calcium hydroxide at ordinary temperatures to form compounds possessing cementitious properties. It is essential that pozzolana be in a finely-divided state as it is only then that silica can combine with calcium hydroxide (produced by the hydrating Portland cement) in the presence of water. Some examples of pozzolans are discussed below: (i)Silica Fume: Silica fume is also referred to as microsilica or condensed silica fume, but the term ‘silica fume’ has become generally accepted. It is a byproduct of the manufacture of Silicon and ferrosilicon alloys from high-purity quartz and coal in a submerged-arc electric furnace. The escaping gaseous Silicon monoxide (SiO) oxidizes and condenses in the form of extremely fine spherical particles of amorphous silica (SiO2); hence, the name silica fume. Silica in the form of glass (amorphous) is highly reactive, and the smallness of the particles speeds up the reaction with calcium hydroxide produced by the hydration of Portland cement. The very small particles of silica fume can enter the space between the particles of cement, and thus improve packing. When the furnace has an efficient heat recovery system, most of the carbon is burnt so that silica fume is virtually free from carbon and is light in colour. Furnaces without a full heat recovery system leave some carbon in the fume, which is therefore dark in colour. (ii)Fly Ash: Fly ash, also known as flue-ash, is one of the residues generated in combustion, and comprises the fine particles that rise with the flue gases. Ash which does not rise is termed bottom ash. In an industrial context, fly ash usually refers to ash produced during combustion of coal. Fly ash is generally captured by electrostatic precipitators or other particle filtration equipment before the flue gases reach the chimneys of coal-fired power plants, and together with bottom ash removed from the bottom of the furnace is in this case jointly known as coal ash. Depending upon the source and makeup of the coal being burnt, the components of fly ash vary considerably, but all fly ash includes substantial amounts of Silicon dioxide (SiO2) (both amorphous and crystalline) and calcium oxide (CaO), both being endemic ingredients in many coal-bearing rock strata. Toxic constituents depend upon the specific coal bed makeup but may include one or more of the following elements or substances in quantities from trace amounts to several percent: arsenic, beryllium, boron, cadmium, chromium, hexavalent chromium, cobalt, lead, manganese, mercury, molybdenum, selenium, strontium, thallium, and vanadium, along with dioxins and Polycyclic aromatic hydrocarbon (PAH) compounds In the past, fly ash was generally released into the atmosphere, but pollution control equipment mandated in recent decades now require that it be captured prior to release. In the US, fly ash is generally stored at coal power plants or placed in landfills. About 43% is recycled, often used to supplement Portland cement in concrete production. Some have expressed health concerns about this. 32 In some cases, such as the burning of solid waste to create electricity ("resource recovery" facilities a.k.a. waste-to-energy facilities), the fly ash may contain higher levels of contaminants than the bottom ash and mixing the fly and bottom ash together brings the proportional levels of contaminants within the range to qualify as nonhazardous waste in a given state, whereas, unmixed, the fly ash would be within the range to qualify as hazardous waste. (iii)Slag: Slag is a partially vitreous byproduct of the process of smelting ore, which separates the desired metal fraction from the unwanted fraction. Slag is usually a mixture of metal oxides and Silicon dioxide. However, slags can contain metal sulfides and metal atoms in the elemental form. While slags are generally used to remove waste in metal smelting and as a pozzolan, they can also serve other purposes, such as assisting in the temperature control of the smelting, and minimizing any re-oxidation of the final liquid metal product before the molten metal is removed from the furnace and used to make solid metal. Benefits of using pozzolan: Lithification: Once the Natural pozzolan-lime mixture is hydrated, the pozzolanic reaction begins immediately and continues for many years. Eventually, the mass will reach complete lithification, forming a rocky material similar to plagioclase with some content of magnetite. The compressive strength as well as the flexural strength will continue to increase for a long time. This unique characteristic is one of the main reasons many great ancient structures have lasted for over two thousand years. Autogenous Healing: A unique characteristic of Natural pozzolan is its inherent ability to actually heal or re-cement cracks within the concrete by means of the continuation of pozzolanic reaction with the calcium hydroxide freed from the cement hydration reaction. This results in the filling up of most of the gaps inside the hardened concrete matrix. Reduced Permeability and Voids: The leaching of water-soluble calcium hydroxide produced by the hydration of Portland cement can be a significant contributor to the formation of voids. The amount of "water of convenience" used to make the concrete workable during the placing process creates permeable voids in the hardened mass. Natural pozzolan can increase the fluidity of concrete without "water of convenience," so that the size and number of capillary pores created by the use of too much water can be minimized. Reduces Expansion and Heat of Hydration: Experiments show that replacing 30% Portland cement with Natural pozzolan can reduce the expansion and heat of hydration to as low as 40% of normal. This may be because there is no heat produced when Natural pozzolan reacts with calcium hydroxide and that the free calcium oxide in the cement can hydrate with natural pozzolan to form C-S-H. Natural pozzolan decreases the heat generated by cement hydration and delays the time of 33 peak temperature. The graphic pattern of Natural pozzolan - Portland cement mixture is extended longer and lowers to form a much more moderate curve than the heat of hydration curve of Portland cement itself. Reduces Creep and Cracks: While concrete is hardening, the "water of convenience" dries away. The surface of the hardening mass then begins to shrink as the temperature goes down from outside. This results in the formation of creep and cracks. Natural pozzolan moderates the expansion and shrinkage of concrete. It also helps to lower the water content of the fresh concrete. Therefore, the creep and cracks can be significantly reduced without the process of water cooling. Reduces Micro cracking: The expansion and shrinkage mentioned above also create micro cracks inside the hardened C-S-H paste and in-between the aggregate and the C-S-H paste. These micro cracks significantly contribute to concrete permeability as well as other concrete defects. The Natural pozzolan- Portland cement mixture expands these shrinks so moderately that there is no micro cracking inside the C-S-H paste after drying. Increases Compressive Strength: The pozzolanic reaction between natural pozzolan and calcium hydroxide happens after the C3S and C2S in the cement begins to hydrate. At the early stage of curing, 30% Natural pozzolan substituting Portland cement mixture is slightly lower than reference OPC [Ordinary Portland Cement} in regard to compressive strength. As time goes by, natural pozzolan continues to react with the calcium hydroxide produced by cement hydration and increases the compressive strength by producing additional C-S-H. After 21 curing days, the 30% Natural pozzolan/ 70% Portland cement mixture begins to exceed reference OPC in compressive strength. After 28 days, it exceeds reference OPC by about 15%. The pozzolanic reaction continues until there is no free calcium hydroxide available in the mass and the compressive strength exceeds the reference OPC by 30-40%. Increases Resistance to chloride Attack: Concrete deterioration caused by the penetration of chloride occurs quickly when chloride ions react with calcium. The expansion of hydrated calcium oxy-chloride enlarges the microcracks and increases the permeability that causes quicker chloride penetration and more damage from freezing and thawing action. The 30% Natural pozzolan added into cement can react with almost all the free calcium hydroxide and form a much denser past. Thus, the penetration of chloride can be minimized and the few penetrated chloride ions cannot find free calcium hydroxide with which to react. Increases resistance to sulphate attack: There are three chemical reactions involved in sulphate attack on concrete: 1) Combination of free calcium hydroxide and sulphate to form gypsum (CaSO4-2H2O). 2) Combination of gypsum and calcium aluminate hydrate (C-A-H) to form ettringite (C3A-3CaSO-32H2O). 3) 34 Combination of gypsum and calcium carbonate with C-S-H to form thaumasite (CaCO3-CaSiO3-CaSO4-15H2O). All these reactions result in the expansion and disruption of concrete. Thaumasite in particular is accompanied by a very severe damaging effect which is able to transform hardened concrete into a pulpy mass. Reduces alkali-aggregate reaction: Because Natural pozzolan is shattered into such a fine particle size resulting in dramatically increased reactive surface area, it can react quickly with calcium hydroxide and can trap the alkali inside the cement paste. Thus, it helps to form a denser paste with almost no alkali-aggregate reaction at all. Protects steel reinforcement from corrosion: The preceding discussions make it very clear that concrete made from 30% Natural pozzolan/ 70% Portland cement mixture can protect steel reinforcement because it creates an environment so densely packed that no liquids or gases can penetrate through it to cause corrosion to the steel. Increases abrasion resistance: Natural pozzolan increases the compressive strength of concrete and makes the concrete matrix stronger and denser. It also prevents the formation of pulpy, crispy, or water-soluble materials created by chemical attack. Therefore, it helps the concrete to durably resist abrasion. Lowers water requirement with high fluidity, self-leveling, and compression: In normal operations, the bulk volume of concrete in the constructions are placed and compacted by use of high frequency poke vibrators. The rapid vibration induces segregation phenomena of all orders of magnitude in the fresh concrete, e.g., stone segregation, internal bleeding giving bonding failures, and inhomogeneous cement paste and air-void systems. Under proper use of vibratory compaction, Natural Pozzolan minimizes or eliminates these problems due to the amorphous structure of the pozzolan particles. Improves Durability: The benefits and characteristics of Natural Pozzolan mentioned above clearly explain why the ancient structures built by the Greeks have survived over 2000 years of weathering. 2.5 Summary Silt is a material being dredged out from various reservoirs where it is a cause of reduction in capacity of reservoir; it has certain physical, chemical and engineering properties. It has numerous uses in engineering as well as industrial sector. Cement is a binding material used in construction; it has a certain chemical composition and can be manufactured from dry process as well as wet process.ASTM has defined various standards for it. Then there are pozzolans which in finely-divided form, in the presence of moisture possess cementitious properties. 35 Chapter 3 Methodology 3.1 Introduction In this chapter the overall track of the project is discussed that how the project is proceeded towards the end results passing trough different experimental stages. Different tests, procedures and mechanisms are discussed briefly that lead to the project output. 3.2 Tests on TD silt First of all the sample collected from TDR analyzed chemically and physically for its different components. This is the prime stage of the project that leads us to the point that the TDR silt possesses both physical and chemical properties to be used as: A cement producer A cement replacement Also the silt contains certain minerals and constituents that can provide economical cement than the market available due to its pre-available constituents required for cement. 3.3 Tests on off-the-shelf cement The cement available in the market was tested with full precautions according to the ASTM Standards for the data availability and for later on comparison. The main tests that performed during the manoeuvre are: 3.3.1 Fineness (ASTM C 184 – 94 Dry method for fineness) In this test a small amount of dry cement was subjected to standard sieve covered with lid for a specified duration. The percent passing and percent retain calculated from weights passed and retained on standard sieve 3.3.2 Consistency (ASTM C 187) In this test the standard cement paste was determined using Vicat’s apparatus. The reading of the apparatus gave the amount of water required to make the standard cement paste 3.3.3 Setting time (ASTM C 191 – 04) According to the standards the initial setting time and final setting time of cement was determined using the same Vicat’s apparatus. The needle and plunger readings for a specified elapsed time were observed that gave the setting time of the cement. 36 3.3.4 Compressive strength (ASTM C 109/ C 109M) The compressive strength tests performed for 7th day and 28th day. According to the standards 2˝ cement mortar cubes were moulded, extracted, cured and tested in UTM to find its compressive strength on its 7th and 28th day 3.4 Cement production from TDR silt From here onwards the next phase of the project starts i.e. the production phase. The objective of this phase is to produce the cement from TDR silt according to the conventional procedures followed by cement industries. The schematic diagram of the process is shown: Analysis Of TD Silt Cooling Clinkers Adding 5% Gypsum To Each Composition Different Compositions For Cement Production Clinkering Of Each Composition (Pallets) Grinding Pallets Formation From Each Composition Drying Pallets Packaging Figure3.1: Schematic diagram of TD Cement Production process 37 3.4.1 Different compositions for cement In this step different compositions of cement were made. The percentage of Chalk (source of Limestone, CaCO3) was changed for every composition. Different compositions formed were: Sample A : Sample B : Sample C : Sample D : TDR Silt with 0% Chalk TDR Silt with 20% Chalk TDR Silt with 30% Chalk TDR Silt with 60% Chalk The samples were thoroughly mixed and then molasses were added as a binder for pallets formation from each composition. The pallets were placed in safe place to dry and make ready for the next step. 3.4.2 Clinkering When the pallets became completely dry, each composition was heated at a temperature of about 1150Cº in a small Rotary Kiln. Each composition subjected to this temperature till the pallets turned into hard clinkers. The pallets fused into each other at that very high temperature resulted in very hard clinker. 3.4.3 Grinding with Gypsum After cooling the clinkers, each composition was subjected to grinding with addition of 5% Gypsum. Each composition was grinded in Rod Mill Grinder to a very fine level. This step actually resulted in an output as a TD cement. Precautions against the air moisture were taken in this step. 3.4.4 Packaging The cement produced from each composition was carefully packed in air tight bags to avoid addition of moisture from the environment. The bags were stored in a dry place. Each bag was marked depending upon their composition and extra details were provided on tags with each bag showing its overall composition. 3.5 Tests on TD cement The cement produced in the process then tested for fineness which gave satisfactory results regarding the fineness. As the main objective of the project circulates around the strength of the cement so according to the same standards as applied for the market available cement, compressive strength tests were performed but in two styles: 3.5.1 As pure cement The same 2” x 2” cubes were made using TD cement according to the ASTM standards, moulded, extracted, cured and then tested in UTM for 7th and 28th day strength. 3.5.2 As cement replacement In this step the cubes were made using 50% market available cement and 50% TD cement. The cubes were made in the same manner according to the ASTM Standards and then tested using the same schedule and procedure. 38 3.6 Summary The overall mechanism of the project is to produce cement from TDR silt with changing its main constituents and testing it according to the ASTM Standards as: Pure cement Cement replacement And then comparing the results with the data obtained from tests on market available cement. Project Market Cement TD Cement Tests as Pure Cement Tests as Cement Replacement Tests as Pure Cement Figure3.2: Schematic diagram showing Project Overview 39 Chapter 4 Experimental work and Results 4.1 Introduction In this chapter we will discuss different techniques used in laboratory and results obtained to check the viability of our project. The chapter comprises of tests conducted on Tarbela Dam (TD) silt, some standard tests on off-the shelf cement sample. We will also discuss the manufacturing of TD cement in laboratory and tests conducted on TD cement both as pure cement and as cement replacement. 4.2 Analysis of TD silt Silt samples obtained from Tarbela Dam Reservoir were analyzed thoroughly to find out the composition of TD silt. Results of tests on TD silt are shown as under: Table4.1: Chemical Composition of TD silt Chemical Composition Parameters Silt Analysis Silica as SiO2 % 53.86 Aluminum as Al2O3 % 16.10 Iron as Fe2O3 % 6.71 Calcium as CaO % 6.66 Magnesium as MgO % 4.31 0.06 SO3 % Sodium as Na2O % 2.25 Potassium as K2O % 3.33 40 Table4.2: Properties of TD silt Physical Properties Fineness All passes sieve no.200 Moisture content Controllable in drainage basin Loss on Ignition (LOI) 8.01% 4.3 Tests on off-the shelf cement Following tests conducted on cement sample available from market are discussed as under. 4.3.1 Fineness by dry process (ASTM C 184 - 94) Fineness of cement is measured by sieving it on standard sieve. The proportion of cement of which the grain sizes are larger than the specified mesh size is thus determined. Calculations are given below. Calculation and Observations: Sieve #200 Shaking time= 15 min Weight of cement=W1= 50 gm Weight of Retained=W2= 4gm Percentage passing of cement= [(W1-W2)/W1]*100 %age passing of cement= [(50-4)/50]*100 Result: Percentage passing of cement = 92% 41 Figure4.1: Retained cement on Sieve #200 4.3.2 Consistency (ASTM C 187) Consistency test was performed in laboratory to find the amount of water to be used to make a workable mix with the given cement sample. According to ASTM C 187: Range of penetration of Plunger=10 +1mm By using Vicat’s apparatus in laboratory the results obtained are given below: Observations and Results: Reading=9 mm Volume of water used=80 ml 4.3.3 Setting time (ASTM C 191 - 04) In the laboratory setting time of the given cement sample was determined using Vicat’s apparatus shown in Figure 4.2.The readings taken and results obtained are given as under: 42 Figure4.2: Vicat's Apparatus Observations and Calculations: Temperature = 14oC Weight of cement = 300gm Water used = 80ml %age of water = 26.66 % Temperature of mixing water = 23.5oC Table4.3: Initial setting time readings S.No. 01 02 03 04 05 06 07 08 09 10 11 12 13 Time interval 15 min 15 min 15 min 15 min 15 min 15 min 15 min 15 min 15 min 15 min 15 min 15 min 15 min 43 Penetration 40mm 40mm 40mm 40mm 40mm 40mm 40mm 40mm 35mm 35mm 30mm 30mm 25mm Results: Initial setting time=2 hr 50 min. Final setting time=5 hr 45 min. 4.3.4 Compressive strength (ASTM C 109/C 109M) To find out the compressive strength of available cement sample the mortar cubes of 2in x 2in were prepares in laboratory. After curing these cubes were tested for 7th day and 28th day compressive strengths. The quantities of ingredients used and results obtained are given as under: Weight of standard sand (passed from sieve #16 and retained on sieve #30) =1375gm Weight of cement=500gm Volume of water=240 ml Figure4.3: Standard Sand The cubes prepared in laboratory are shown in Figure 4.4. 44 Figure4.4: Two moulds containing three cubes each Results: 7th day Compressive strength = 2117 psi 28th day Compressive strength = 2733 psi 4.4 Production of TD cement in Laboratory Production of cement from Tarbela Dam Reservoir (TDR) silt comprises mainly of three phases discussed as under: In first phase four different compositions were prepared by making pellets from silt and chalk used as a source of limestone (CaCO3) using molasses as a binder. Sample A weights used: Weight of Silt = 4.80Kg Weight of Chalk = not used Sample B weights used: Weight of Silt = 3.60Kg Weight of Chalk = 0.72Kg Total weight of Sample = 4.32Kg Sample C weights used: Weight of Silt = 7.0Kg Weight of Chalk = 3.0Kg Total weight of Sample = 10Kg 45 Sample D weights used: Weight of Silt = 4.0Kg Weight of Chalk = 6.0Kg Total weight of Sample = 10Kg Percentages of silt and chalk contained by each sample are given in table 4.4. Table4.4: Different Compositions with TDR silt Sample Silt Chalk A 100 % 0% B 80 % 20 % C 70 % 30 % D 40 % 60 % Hand mixing and pellet formation from samples is shown in Figure 4.5, 4.6 and 4.7 46 Figure4.5: Hand mixing of TDR Silt and Chalk Figure4.6: Adding Molasses to the sample Figure4.7: Pellets from the sample In second phase of cement production from TDR silt, completely dried pallets of each composition were heated in a small rotary kiln available in the laboratory at a temperature of about 1150oC. The clinkers are the allowed to cool. 47 Figure4.8: Rotary kiln in laboratory Figure4.9: Clinkers produced In the final phase the clinkers were grounded with 5% gypsum to produce cement from each composition of fineness enough to pass sieve #200 as prescribed by ASTM. 48 Figure4.10: Rod Mill Grinder in laboratory 4.4 Tests on TD cement To analyze TD cement produced in laboratory the compressive strength test was conducted. TD cement was used both as pure cement as well as 50% replacement of cement available in the market using each and every sample produced previously. 4.4.1 As pure cement Using only TD cement and sand in the presence of water results for all the four samples are discussed as under: Sample A: No binding properties were observed when mortar cubes were demoulded after 24 hours of casting time. Sample B: After demoulding the cubes were broken into pieces as shown in figure 4.11. 49 Figure4.11: Broken cubes after Demoulding Sample C: Sample showed some binding property but had not maintained its shape during curing. Sample D: Unlike A, B and C this sample gave some encouraging results. Results obtained for 7 day and 28thday compressive strength of each cube are given in table 4.5. th Table4.5: Compressive strength test readings of Sample D as pure cement S.No. 7th day 28thday 1 876.09 psi 820.99psi 2 898.13 psi 903.64psi 3 776.91 psi 859.56psi Mean 7thday strength = 850.38 psi Mean 28thday strength = 861.39psi 50 Mortar cubes obtained are shown figure 4.12 as under: Figure4.12: TD Cement mortar cubes from sample D 4.4.2 As Cement replacement Using 50% Cement off-the shelf, 50% TD cement and sand in the presence of water results for all the four samples are discussed as under: Sample A: There was some improvement and mortar cubes gave the following results shown in the table 4.6. Table4.6: Compressive strength tests readings of sample A as cement replacement S.No. 7th day 28thday 1 236.93 psi 236.93Psi 2 110.2 psi 242.44Psi 3 154.28 psi 269.99 psi Mean 7thday strength = 167.14 psi Mean 28thday strength = 249.79 psi Mortar cubes obtained are shown figure 4.13 as under: 51 Figure4.13: TD Cement mortar cubes from Sample A Sample B: With 50% cement off-the shelf 50% TD cement gave the following results as given in table 4.7. Table4.7: Compressive strength tests readings of sample B S.No. 7th day 28thday 1 655.69 psi 732.83Psi 2 613.97 psi 771.40Psi 3 600.59 psi 909.15psi Mean 7thday strength = 623.42 psi Mean 28thday strength = 804.46 psi Mortar cubes obtained are shown figure 4.14 as under: 52 Figure4.14: TD Cement mortar cubes from Sample B Sample C: The compressive strength of each mortar cube recorded is given in table 4.8. Table4.8: Compressive strength tests readings of Sample C as cement replacement S.No. 7th day 28thday 1 1978.09psi ----- 2 2159.92psi ----- 3 1289.34psi ----- Mean 7th day strength = 1809.11 psi Mean 28thday strength = Result awaiting Sample D: Compressive strength results obtained for each cube are given in table 4.9. Table4.9: Compressive strength tests readings of Sample D S.No. 7th day 28thday 1 876.09psi ----- 2 820.99psi ----- 3 914.66psi ----- 53 Mean 7th day strength = 870.58 psi Mean 28thday strength = Result awaiting Figure 4.15 shows mortar cubes made of sample C and sample D as given below. Figure4.15: Mortar cubes made of sample C (marked as I) and sample D (marked as II) 4.5 Summary In this chapter all the experimental work performed in the laboratory and results obtained are tabulated as well as figurative description is made to present a clear view of the project work. Results regarding Silt analysis, Tests on off-the shelf cement and TD Cement are provided. 54 Chapter 5 Discussion 5.1 Introduction In this chapter we will discuss the results obtained from different tests performed in laboratory on TDR silt, off-the shelf cement and TD cement produced in laboratory. 5.2 Analysis of Tarbela Dam Reservoir (TDR) silt Analysis of TDR silt in laboratory indicated the presence of different natural minerals as shown in the figure 5.1: Composition % 53.86 16.1 6.66 4.31 0.06 SiO2 Al203 CaO MgO SO3 2.25 3.33 Na2O K2O 6.71 6.72 Fe2O3 other Figure5.1: TDR Silt Composition 5.3 Tests on off-the shelf cement Results obtained from different tests on off-the shelf cement sample are discussed as under. Fineness: Fineness of the cement sample recorded was 92% which is greater than 90% according to ASTM C 184 – 94. Thus the cement fineness is within an acceptable range. 55 Consistency: To make a workable mortar from cement and sand volume of water used in consistency determination was noted as 80ml, for which penetration of plunger recorded was 9mm. According to ASTM C 187 consistency of the cement sample is fine. Setting Time: (i) Initial set: Occurs when the paste begins to stiffen considerably. (ii) Final set: Occurs when the cement has hardened to the point at which it can Sustain some load. Setting time of the cement sample recorded was: Initial setting time =2 hr 50 min ≥ 45 minutes Final setting time =5 hr 45 min ≤ 375 minutes Setting time both initial and final is O.K. according to ASTM C 191 – 04. Compressive strength: The compressive strength recorded by testing cement mortar cubes is: 7th day Compressive strength = 2117 psi 28th day Compressive strength = 2733 psi ASTM C 109/C 109M States that compressive strength of mortar cube should not be less than 1800 psi. Thus the compressive strength is O.K. 5.4 Tests on TD Cement There were four samples of cement produced from Tarbela Dam silt in the laboratory. Each sample contained TD silt (containing Silica, Aluminum and Magnesium etc.) as a raw material and Chalk as a source of limestone (CaCO3). These proportions can be shown as: 56 Sample A Chalk 0% TD Silt 100% Figure5.2: Sample A composition Figure5.3: Sample B Composition 57 Sample C Chalk 30% TD Silt 70% Figure5.4: Sample C Composition Sample D TD Silt 40% Chalk 60% Figure5.5: Sample D Composition 58 These entire TD Cement samples were tested for compressive strength analysis and the results obtained were: (a) TD Cement used as pure cement in mortar: Table5.1: TD Cement Compressive strength as pure cement Sample 7th day strength 28th day strength A ------ ------ B ------ ------ C ------ ------ D 850.38 psi 861.39psi (b) TD Cement used as a Cement replacement: Table5.2: TD Cement compressive strength as 50% off-the shelf cement replacement Sample 7th day strength 28th day strength A 167.14 psi 249.79 psi B 623.42 psi 804.46 psi C 1809.11 psi ----- D 870.58 psi ----- 5.6 Summary In this chapter we discussed composition of Tarbela Dam Reservoir silt, Tests on off-the shelf cement, Compressive strength tests on TD Cement and results obtained in the laboratory. Different compositions of TD cement are represented as graphical data. Compressive strength of each TD sample is provided. 59 Chapter 6 Conclusions 6.1 Introduction This chapter is the essence of the whole project. The findings from the project and the fruitful effects directly and indirectly after its application and its impacts on environment, development and society are discussed. 6.2 Conclusions Nothing is perfect in this world. Every work starts from a start point considered as a zero point of the work. To climb a peak, a start is mandatory. The conclusions that are derived from the project are: 6.2.1 TD Cement Mortar mix Following ASTM standards the minimum required compressive strength of the mortar types are: Type M 2,500 psi Type S 1,800 psi Type N 750 psi Type O 350 psi Inference: As already discussed earlier compressive strength of Sample D of TD Cement mortar is 860 psi(refer to Table 4.5) and when 50%Cement off-the shelf is replaced compressive strength of mortar is 804 psi (refer to Table 4.8). Thus TD Cement can be a substitute for Type N and Type O mortar mix in construction industry. All of the above discussion can be concluded in terms of comparing Compressive strengths of Mortar mix Type N, Type O and TD Cement as shown in figures 6.1 and 6.2. 60 Compressive strengths (psi) Mortar Mix O Mortar Mix N TD Cement Mortar 850.38 750 750 350 750 350 350 0 350 0 Sample A 750 0 Sample B Sample C Sample D Figure6.1: TD Cement mortar mix Vs Type N and Type O mortar mix Compressive strengths(psi) Mortar Mix O 750 750 350 Mortar Mix N TD Cement Mortar 804.46 750 350 350 750 350 249.79 Sample A Sample B Sample C Sample D Figure6.2: 50% TD Cement +50% off-the Shelf Cement mortar mix Vs Type N and Type O mortar mix 6.2.2 Low Energy Cement TD Cement is manufactured at 1150C˚. The cement industries produce Ordinary Portland Cement (OPC) at temperature above 1400C˚. This difference of heat saves a nominal amount of energy which makes it more economical cement than the market available cement. 61 The energy consumed is in form of natural gas, its considerable amount can be saved for this approximately 300C˚ difference. This saving in industrial use can make considerable change, as small units combine to form a large structure. The heating systems used are also very expensive because to withstand that high temperature, the materials used should have great capacity to resist that large amount of heat. By decrease of that heating temperature, the demand of the resisting capacity of the material will also decrease and ultimately the savings in terms of machinery cost can also be made. 6.2.3 Storage capacity of TDR According to official documents, the usable storage capacity at the time of Tarbela’s commissioning was 9.68 million-acre feet (MAF), which has now reduced to 6.77 MAF due to sedimentation and is continuously on the decline. By dredging the heavy amount of silt that can be further used as a raw material for cement production will result in retaining the original storage capacity up to much extent. The increase in storage capacity will not only improve the Hydro Electric Power sector but also irrigation, flood storage and control. 6.2.4 Revenue to accommodate heavy costs of dredging The heavy costs of dredging can be reduced by finding an industrial use of the silt in the reservoir. In reference to the project under discussion production of cement from Tarbela Dam Reservoir silt as a raw material will be a good option to produce revenue and decrease the cost of dredging silt from the reservoir. 6.2.5 Environmental protection The dumping of dredged material will also be a problem. As the nature of silt is such that its consolidation takes a very long time and its compaction is also an issue, therefore its use like other soil types as a filling material is not appreciated. The use of the dredged material as a cement producer will also be an act of environmental protection. Otherwise dumping this material at different sites will be a serious challenge for the environment. 6.2.6 An idea of introducing cement plants in TD locality In TD locality there are certain sites which can perform as suitable locations for introducing cement plants. This will be a good idea in a sense that the raw material will be easily available at a very economical rate. The transportation charges of the raw material will be minimized as much as possible. 6.2.7 Employment By introducing cement plants in the locality employment chances especially for that area will increase automatically. Similarly the dredging process will also be on a large scale so it will also offer many employment chances during the whole tenure of the dredging project. 62 6.2.7 Business competition It is obvious that whenever a new product is introduced in market, a competition starts in market, as a result quality increases and prices decrease and the user gets advantage. 6.3 Summary The project is not only just producing cement from silt. It will also help in: Increasing the storage capacity of TDR. Producing low energy cement. Introducing a new product in to material sciences. Earning revenue to minimize cost of dredging process. Protection of the environment by eliminating freely dumping of dredged material into the environment. Revival of waterways of Pakistan. 63 Chapter 7 Future plans 7.1 Introduction In this chapter those aspects of the project are discussed which can make the project more viable to meet the requirements of the standards. Those angles are clearly mentioned which are worth to try and probably can give good results regarding the objective and scope of the project. 7.2 Future recommendations The project results can be improved up to the mark by introducing more sophistication to the procedures and tests so that good results can be achieved. The angles of the project which are worth to improve are: 7.2.1 Temperature The recommended temperature for the clinkering process is 1300-1400Cº, above which calcinations occur and as a result the particles are fused in such a manner that result in further reactions for achieving the strength with addition of water to it. This temperature is very difficult to achieve in the small furnace on a small level. The temperature achieved in the lab was hardly 1150Cº, so there may be a possibility that the calcinations and fusion of the particles did not occur that is the required for the standard reactions of cement particle and water to give the desired strength that is set by the standards. So it is expected that if the temperature issue is overwhelmed then there may be nominal changes in the results that are desired. 7.2.2 Silt sampling The reservoir is about 97 square miles and the sample collected from a specific location is not necessarily same as the sample collected from the other locations chemically within the same reservoir. There should be a proper mechanism of silt sampling. Such stations of sampling should be allocated where the sampling procedure is easy, economical and technically feasible. The most important point is that where it is obvious that the chemical composition of all the silt with in the vicinity of the station does not vary. 7.2.3 Microscopic study of silt samples Each sample should be analyzed chemically for each component. The change in the components of the sample within the same reservoir for each sampling location should also be determined because introducing the same procedure for samples with different chemical properties may be a challenge. 64 X-ray analysis of each sample should also be carried so that accurate results are achieved and both the analysis results can be compared. So from both analysis there will be a proper list of chemical composition of silt and its variation with change of sampling station with in the same reservoir. 7.2.4 Microscopic study of the cement produced The same analysis (i.e. both chemical and x-ray) should be carried out for the cement samples produced so that the exact information of the cement particles, their shapes and distribution are known. The results obtained should be then compared with the market available cement to know the exact nature and information regarding the cement. From this study, the exact structural arrangement of the particles and composition of each sample can give much help if a sample does not give the desired strength and other properties i.e. the exact reason can easily and directly be investigated. 7.2.5 Using sophisticated instruments For every experimental work instruments standard plays an important role in the final results. The kiln used for the firing purpose was not purely designed and used for cement earlier, it was used for the production of other compounds like zinc sulphate, zinc carbonate etc. Due to high temperature the material particles fuse with the kiln itself and then harden after cooling. The material remains within the kiln and when the same kiln is used for the clinkering purpose those foreign particles also fuse at high temperature with the cement particles and even can change the chemical composition. Therefore this issue should be eliminated. Similarly in grinding phase the grinding machine should be used purely for cement or should be clean enough that no foreign particles add in cement. 7.2.6 Controlled environment In cement industry there is controlled environment during the production process. During and after grinding phase the moisture has a great influence on cement, the packaging of the samples should be done in a controlled environment. 7.2.7 Research for additives The different samples of cement produced were just worked out for the addition and effect of variation of CaO, certain additives should be identified that are cheap and can improve the strength and other properties. Such mineral should be searched that can increase the performance level of the cement. Such pozzolans should be investigated that can give better results to the strength and that are cheap and easily available. 7.2.8 A special phenomenon A phenomenon observed when the cubes were placed in water for curing, as they were placed, a special type of reaction started, liberating bubbles in water and ultimately the cubes changed to fine aggregate and the cement dissolved in water, 65 The nature of the bubbles and its reason should be investigated whether it is a special type of gas that is liberated by the reaction which can further lead to the type of reaction or it is heat of hydration in the form of bubbles 7.2.9 Study the effects of alkalis present and their elimination The silt contains certain alkalis that may be a problem in case of massive concrete structure because they account for alkali expansion. Certain processes should be worked out to eliminate these compounds, because they cause serious problems in massive structures like dams etc. 7.3 Summary If the above ideas are introduced in the procedures and precautionary measures then it is expected that the results will touch the desired values of the standards and may differ positively from the present scenario or may be the results will be more fruitful than expected. 66 Appendix-A ASTM Standards for Cement and Concrete Designation C418 - 12 Abrasion Testing Title Standard Test Method for Abrasion Resistance of Concrete by Sandblasting C779 / C779M Standard Test Method for Abrasion Resistance of Horizontal Concrete Surfaces 12 C944 / C944M - Standard Test Method for Abrasion Resistance of Concrete or Mortar Surfaces by 12 the Rotating-Cutter Method C1138M - 12 Standard Test Method for Abrasion Resistance of Concrete (Underwater Method) Additions C226 - 12 Standard Specification for Air-Entraining Additions for Use in the Manufacture of Air-Entraining Hydraulic Cement C465 - 10 Standard Specification for Processing Additions for Use in the Manufacture of Hydraulic Cements C688 - 08 Standard Specification for Functional Additions for Use in Hydraulic Cements C1565 - 09 Standard Test Method for Determination of Pack-Set Index of Portland Cement Air Entrainment C185 - 08 Standard Test Method for Air Content of Hydraulic Cement Mortar Application of Exterior Insulating and Finish Systems and Related Products C1397 - 09 Standard Practice for Application of Class PB Exterior Insulation and Finish Systems (EIFS) and EIFS with Drainage C1516 05(2011) Standard Practice for Application of Direct-Applied Exterior Finish Systems C1535 05(2011) Standard Practice for Application of Exterior Insulation and Finish Systems Class PI 67 Designation Chemical Admixtures Title C233 / C233M 11 Standard Test Method for Air-Entraining Admixtures for Concrete C260 / C260M 10a Standard Specification for Air-Entraining Admixtures for Concrete C403 / C403M 08 Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance C494 / C494M 13 Standard Specification for Chemical Admixtures for Concrete C796 / C796M 12 Standard Test Method for Foaming Agents for Use in Producing Cellular Concrete Using Preformed Foam C869 / C869M 11 Standard Specification for Foaming Agents Used in Making Preformed Foam for Cellular Concrete C979 / C979M 10 Standard Specification for Pigments for Integrally Colored Concrete C1017 / C1017M - Standard Specification for Chemical Admixtures for Use in Producing Flowing 07 Concrete C1582 / C1582M - Standard Specification for Admixtures to Inhibit Chloride-Induced Corrosion of 11 Reinforcing Steel in Concrete C1622 / C1622M Standard Specification for Cold-Weather Admixture Systems 10 Chemical Reactions C227 - 10 Standard Test Method for Potential Alkali Reactivity of Cement-Aggregate Combinations (Mortar-Bar Method) C289 - 07 Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Chemical Method) C441 / C441M - 11 Standard Test Method for Effectiveness of Pozzolans or Ground Blast-Furnace Slag in Preventing Excessive Expansion of Concrete Due to the Alkali-Silica Reaction C586 - 11 Standard Test Method for Potential Alkali Reactivity of Carbonate Rocks as Concrete Aggregates (Rock-Cylinder Method) C1105 - 08a Standard Test Method for Length Change of Concrete Due to Alkali-Carbonate Rock Reaction 68 Title Designation C1260 - 07 Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method) C1293 - 08b Standard Test Method for Determination of Length Change of Concrete Due to Alkali-Silica Reaction C1567 - 13 Standard Test Method for Determining the Potential Alkali-Silica Reactivity of Combinations of Cementitious Materials and Aggregate (Accelerated Mortar-Bar Method) Chemical Tests C25 - 11 Standard Test Methods for Chemical Analysis of Limestone, Quicklime, and Hydrated Lime C400 98(2006) Standard Test Methods for Quicklime and Hydrated Lime for Neutralization of Waste Acid C1164 92(2009) Standard Practice for Evaluation of Limestone or Lime Uniformity From a Single Source C1271 99(2012) Standard Test Method for X-ray Spectrometric Analysis of Lime and Limestone C1301 95(2009)e1 Standard Test Method for Major and Trace Elements in Limestone and Lime by Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP) and Atomic Absorption (AA) C1318 95(2009)e1 Standard Test Method for Determination of Total Neutralizing Capability and Dissolved Calcium and Magnesium Oxide in Lime for Flue Gas Desulfurization (FGD) 69 Designation Compositional Analysis Title C114 - 11b Standard Test Methods for Chemical Analysis of Hydraulic Cement C1356 07(2012) Standard Test Method for Quantitative Determination of Phases in Portland Cement Clinker by Microscopical Point-Count Procedure C1365 06(2011) Standard Test Method for Determination of the Proportion of Phases in Portland cement and Portland-Cement Clinker Using X-Ray Powder Diffraction Analysis Concrete's Resistance to Fluid Penetration C642 - 13 Standard Test Method for Density, Absorption, and Voids in Hardened Concrete C1202 12 Standard Test Method for Electrical Indication of Concrete's Ability to Resist Chloride Ion Penetration C1543 10a Standard Test Method for Determining the Penetration of Chloride Ion into Concrete by Ponding C1556 11a Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion C1585 13 Standard Test Method for Measurement of Rate of Absorption of Water by HydraulicCement Concretes C1760 12 Standard Test Method for Bulk Electrical Conductivity of Hardened Concrete 70 Designation Coordination of Standards Title C183 - 08 Standard Practice for Sampling and the Amount of Testing of Hydraulic Cement C490 / C490M - 11 Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete C511 - 09 Standard Specification for Mixing Rooms, Moist Cabinets, Moist Rooms, and Water Storage Tanks Used in the Testing of Hydraulic Cements and Concretes C778 - 12 Standard Specification for Standard Sand C1005 - 10 Standard Specification for Reference Masses and Devices for Determining Mass and Volume for Use in the Physical Testing of Hydraulic Cements C1222 - 09 Standard Practice for Evaluation of Laboratories Testing Hydraulic Cement Editorial and Nomenclature C51 - 11 Standard Terminology Relating to Lime and Limestone (as used by the Industry) Evaluation of Data (Joint C09 and C01) C670 - 10 Standard Practice for Preparing Precision and Bias Statements for Test Methods for Construction Materials C802 - 09a Standard Practice for Conducting an Interlaboratory Test Program to Determine the Precision of Test Methods for Construction Materials C1067 - 12 Standard Practice for Conducting a Ruggedness Evaluation or Screening Program for Test Methods for Construction Materials C1451 - 11 Standard Practice for Determining Uniformity of Ingredients of Concrete From a Single Source Evaluation of Laboratories C1077 13a Standard Practice for Agencies Testing Concrete and Concrete Aggregates for Use in Construction and Criteria for Testing Agency Evaluation 71 Designation Fineness Title C115 - 10 Standard Test Method for Fineness of Portland Cement by the Turbidimeter C188 - 09 Standard Test Method for Density of Hydraulic Cement C204 - 11 Standard Test Methods for Fineness of Hydraulic Cement by Air-Permeability Apparatus C430 - 08 Standard Test Method for Fineness of Hydraulic Cement by the 45-μm (No. 325) Sieve C786 / C786M - 10 Standard Test Method for Fineness of Hydraulic Cement and Raw Materials by the 300-μm (No. 50), 150-μm (No. 100), and 75-μm (No. 200) Sieves by Wet Methods Ground Slag C989 / C989M 12a Standard Specification for Slag Cement for Use in Concrete and Mortars C1073 - 12 Standard Test Method for Hydraulic Activity of Slag Cement by Reaction with Alkali 72 Bibliography 1. Tippetts-Abbett-McCarthy-Stratton Consulting Engineers, “Tarbela Dam Project Completion Report on Design and Construction”, 1984. 2. Pakistan Water and Power Development Authority, “Annual Report 1999-2000”, a. 2001. 3. Engr. Dr Izhar ul Haq, “Barrages and Dams in Pakistan” for Pakistan Engineering a. Congress, 1990. 4. Dr. Bashir A Chandio and Ms Nuzhat Yasmin, “Proceedings of the National a. Workshop on Water Resources Achievements and Issues in 20th Century and b. Challenges for the Next Millennium”, Pakistan Council of Research in Water c. Resources, June 1999. 5. Asim R. Khan, M. Kaleem Ullah, Saim Muhammad, “Water Availability and Some a. Macro Level Issues Related to Water Resources Planning and Management in the b. Indus Basin Irrigation System in Pakistan”, 2002. 6. Centre of Excellence in Water Resources Engineering, Lahore, “Proceedings - Water for the 21st Century: Demand, Supply, Development and Socio- Environmental a. Issues”, June 1997. 7. Asian Development Bank – TA, Water Resources Sector Strategy, “National Water Sector Profile”, April 2002 8. Dr. Nazir Ahmad, “Water Resources of Pakistan”, Miraj uddin Press, Lahore a. September 1993. 9. Asianics, “A Case Study of Tarbela Dam”, a report for the World Commission on a. Dams, 2001. 10. AM-Neville-properties of concrete 5th edition 11. Mehta, P.K. (1987). "Natural pozzolans: Supplementary cementing materials in concrete". CANMET Special Publication 86: 1–33. 12. Snellings, R.; Mertens G., Elsen J. (2012). "Supplementary cementitious materials". Reviews in Mineralogy and Geochemistry 74: 211–278 13. Cook D.J. (1986) Natural pozzolanas. In: Swamy R.N., Editor (1986) Cement Replacement Materials, Surrey University Press, p. 200. 14. Naik, T.R. and Moriconi, G. (2005) Environment-Friendly Durable Concrete Made with Recycled Materials for Sustainable Concrete Construction. Sustainable Development of Cement, Concrete, and Concrete Structures, Proc. Three day intern, symposium, Toronto, Canada. 5-7 October 2005. 485-505. 15. Dewan, S. (2008) Coal Ash Spill Revives Issue of Hazards. New York Times 16. Tarbela Dam Annual Sedimentation Reports, 2005. 73 17. Soil-Cement Laboratory Handbook, Portland cement Association, EB052, 1992. 18. Soil-Cement Construction Handbook, Portland cement Association, EB003, 1995. 19. ASTM Standard C618, 2004, "Coal, and Fly Ash," ASTM International, Kansas City, Missouri, PA, 2004, DOI: 10.1520/C618, www.astm.org. 20. ASTM Standard C150/C150M, 2004-01, "Specifications for Portland Cement," ASTM International, Kansas City, Missouri, PA, 2004, DOI: 10.1520/C0150-C0150M-04, www.astm.org. 21. PS 232, 1983(R)," Specifications for Portland Cement,” Pakistan Standards, Karachi, PA, 1983(R) 22. University Corporation for Atmospheric Research, 2006 Available at: <http://wegc203116.unigraz.at/meted/hydro/basic/Runoff/print_version/04soilproperties.htm> [Accessed 17 June 2013] 23. Dr. Jeff Thomas and Dr. Hamlin Jennings, The science of concrete, 2008 Available at: <http://iti.northwestern.edu/cement/monograph/Monograph3_8.html> [Accessed 18 June 2013] 24. Civil Engineering Lectures, 2013 Available at: <http://lecture.civilengineeringx.com/traditional-materials/types-of-cement/> [Accessed 18 June 2013] 25. CEMEX S.A.B. de C.V., 2013,Available through: <http://www.cemex.com/ProductsServices/CementTypes.aspx> [Accessed 19 June 2013] 74 75