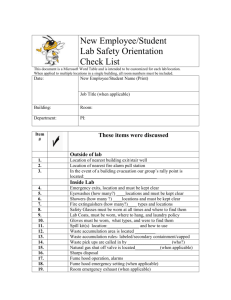
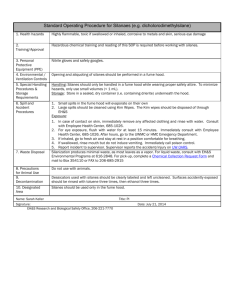
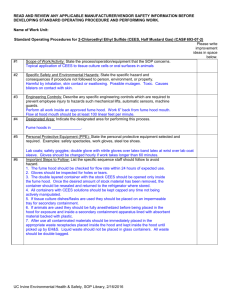
Experiment_23-3a
advertisement

Safety: Consider all compounds to be irritants and toxic. If any of the compounds contact your skin, you should rinse with cool water for fifteen minutes. CO is very poisonous and since it is being released in the reaction the reaction must be carried out in the fume hood and the reaction must be allowed to stay in the fume hood for an adequate period following the reaction. Do not breath the fumes. Dump all residues and do all washing in the fume hood. Generally do all work in the fume hood with the door down at an effective level. Wear gloves, goggles, aprons and appropriate clothing, including closed shoes while carrying out all procedures. Experiment 23: Synthesis of Tetraphenyl-1,2-Dihydrophthalic Anhydride In this experiment tetraphenyl-1,2-dihydrophthalic anhydride will be prepared via a Diels-Alder reaction followed by loss of carbon monoxide. The reaction and mechanism are outlined below. You can use this as a model for your total synthesis project. Think about how you would have to modify the Diels-Alder to obtain hexaphenylbenzene using tetraphenylcyclopentadienone as one of the reagents. This reaction is a good model for the formation of the central benzene ring of hexaphenylbenzene because the second electrocyclic reaction is driven by the loss of carbon monoxide (a leaving group that leaves as a gas) and the formation of the diene in the central ring which is highly conjugated. Obviously you will not be doing this exact reaction, but you will be doing something very similar. Do a retro-electrocyclic reaction to starting with hexaphenylbenzene to the intermediate still containing the strained carbonyl. Then you should be able to see the retro Diels-Alder. Again, your synthesis can be completed using this sort of reaction. This will give you an idea about how you can use a known reaction to predict a related reaction. The intermediate product, I, can exist in two possible stereochemistries, exo or endo shown below. The endo configuration is favored in this reaction. Now as far as your modeling for your total synthesis goes, consider the following……. Procedure 1. Mix 3.5 grams of tetraphenylcyclopentadienone (prepared by student previously via aldol condensation reactions) and 0.95 g of dry maleic anhydride thoroughly in a 100 mL round bottom flask. Add 2.5 mL of bromobenzene to the round bottom. This will result in a thick mixture of solids. A reflux condenser is then attached to the flask and is attached as normal to water hoses (red tubing, water going in the bottom of the condenser and out the top of the condenser into the cup sink in the hood). 2. Since CO (carbon monoxide) will be produced in the reaction, it is necessary to set up the reaction in a fume hood or attach a CO trap. Please see your TA or instructor if for some reason you are not working in a fume hood. 3. When the apparatus is assembled, the mixture is refluxed for a total of three hours until the dark brown color of the solution disappears and a light, yellow solid mass appears. Agitate the mixture occasionally during the reflux period. 4. When the reaction appears to be complete, cool the flask to room temperature and add 25 mL of hexanes. Using a spatula, break up the solid into a finely divided solid in the hexanes and vacuum filter the mixture. Wash the solid with two 5 mL portions of hexanes and let it dry. Take the melting point and then recrystallize the solid from an appropriate solvent system, followed by vacuum filtration and drying. Weigh the purified product and determine its melting point. Measure the IR spectrum for the product as a film or a KBr pellet.

![Safety%20Step%20by%20Step[1]. - thsicp-23](http://s3.studylib.net/store/data/009097871_1-7065ff837c3ce091dcdb4239bcbcbee5-300x300.png)