homepage/sscott/file/Stoichiometry%20Review - Parkway C-2
advertisement
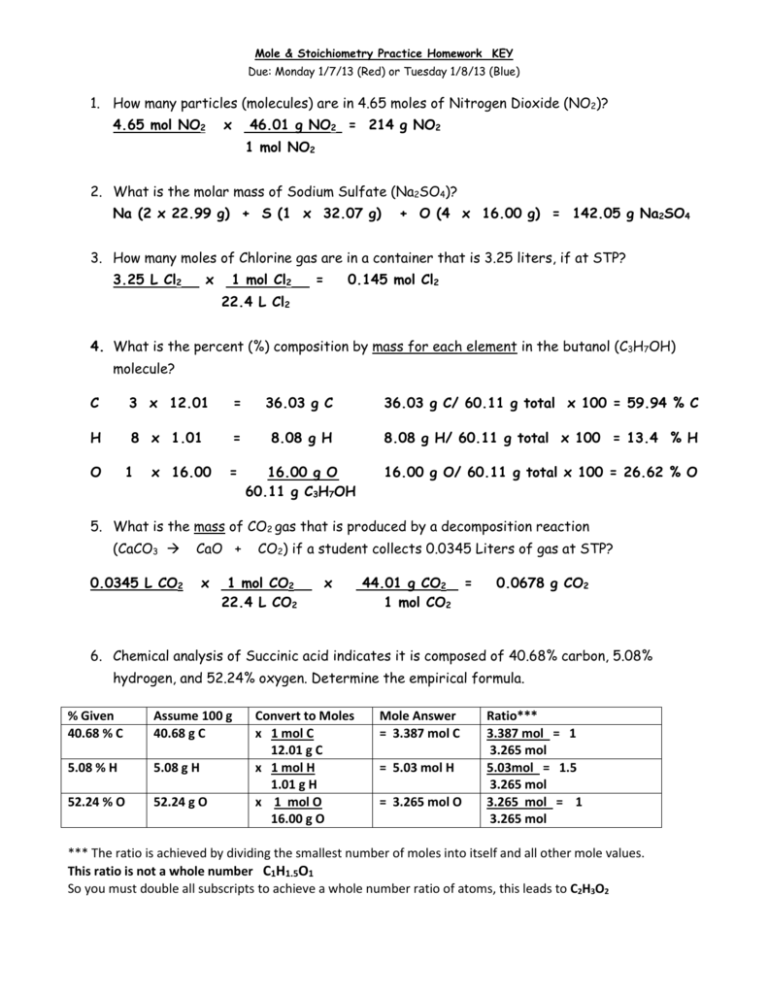
Mole & Stoichiometry Practice Homework KEY Due: Monday 1/7/13 (Red) or Tuesday 1/8/13 (Blue) 1. How many particles (molecules) are in 4.65 moles of Nitrogen Dioxide (NO2)? 4.65 mol NO2 x 46.01 g NO2 = 214 g NO2 1 mol NO2 2. What is the molar mass of Sodium Sulfate (Na2SO4)? Na (2 x 22.99 g) + S (1 x 32.07 g) + O (4 x 16.00 g) = 142.05 g Na2SO4 3. How many moles of Chlorine gas are in a container that is 3.25 liters, if at STP? 3.25 L Cl2 x 1 mol Cl2 = 0.145 mol Cl2 22.4 L Cl2 4. What is the percent (%) composition by mass for each element in the butanol (C3H7OH) molecule? C 3 x 12.01 = 36.03 g C 36.03 g C/ 60.11 g total x 100 = 59.94 % C H 8 x 1.01 = 8.08 g H 8.08 g H/ 60.11 g total x 100 = 13.4 % H = 16.00 g O 60.11 g C3H7OH 16.00 g O/ 60.11 g total x 100 = 26.62 % O O 1 x 16.00 5. What is the mass of CO2 gas that is produced by a decomposition reaction (CaCO3 CaO + 0.0345 L CO2 x CO2) if a student collects 0.0345 Liters of gas at STP? 1 mol CO2 22.4 L CO2 x 44.01 g CO2 = 1 mol CO2 0.0678 g CO2 6. Chemical analysis of Succinic acid indicates it is composed of 40.68% carbon, 5.08% hydrogen, and 52.24% oxygen. Determine the empirical formula. % Given 40.68 % C Assume 100 g 40.68 g C 5.08 % H 5.08 g H 52.24 % O 52.24 g O Convert to Moles x 1 mol C 12.01 g C x 1 mol H 1.01 g H x 1 mol O 16.00 g O Mole Answer = 3.387 mol C = 5.03 mol H = 3.265 mol O Ratio*** 3.387 mol = 1 3.265 mol 5.03mol = 1.5 3.265 mol 3.265 mol = 1 3.265 mol *** The ratio is achieved by dividing the smallest number of moles into itself and all other mole values. This ratio is not a whole number C1H1.5O1 So you must double all subscripts to achieve a whole number ratio of atoms, this leads to C2H3O2 7. If the molar mass of Succinic acid is 118.1 g/mol, what is its molecular formula? Molar Mass = 118.1 g/mol Empirical Mass = 2 so double the subscripts C4H6O4 59.05 g/mol 8. When 104.5 g of Iron (III) Oxide reacts with an excess or carbon monoxide during a laboratory experiment and 54.6 g of Iron is collected by the lab student according to the following equation: Fe2O3 (s) + 3 CO (g) → 2 Fe (s) + 3 CO2 (g) What is the percent yield of the experiment? 104.5 g Fe2O3 x 1 mol Fe2O3 159.70 g Fe2O3 Experimental x 100 = % Yield Theoretical x 2 mol Fe x 55.85 g Fe = 73.09 g Fe 1 mol Fe2O3 1 mol Fe 54.6 g Fe x 100 = 74.7 % Yield 73.09 g Fe 9. Consider the following reaction: 3 NH4NO3 + Na3PO4 (NH4)3PO4 + 3 NaNO3 We have 30.00 grams of ammonium nitrate and 50.00 grams of sodium phosphate determine the following (use dimensional analysis to prove your answer): What is the maximum amount of Ammonium Phosphate that can be formed? 30.00 g (NH4)(NO3) x 1 mol (NH4)(NO3) x 1 mol (NH4)3(PO4) x 149.12 g (NH4)3(PO4) = 80.06 g (NH4)(NO3) 3 mol (NH4)(NO3) 1 mol (NH4)3(PO4) = 18.63 g (NH4)3(PO4) 50.00 g Na3PO4 x 1 mol Na3PO4 x 1 mol (NH4)3(PO4) x 149.12 g (NH4)3(PO4) = 163.94 g Na3PO4 1 mol Na3PO4 1 mol (NH4)3(PO4) = 45.48 g (NH4)3(PO4) What is the limiting reagent? (NH4)(NO3) What is the excess reagent? Na3(PO4)








