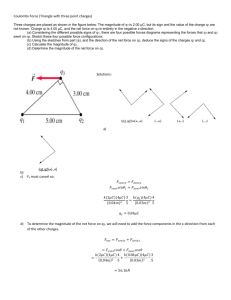
How many excess electrons can be counted in a rubber rod of
advertisement
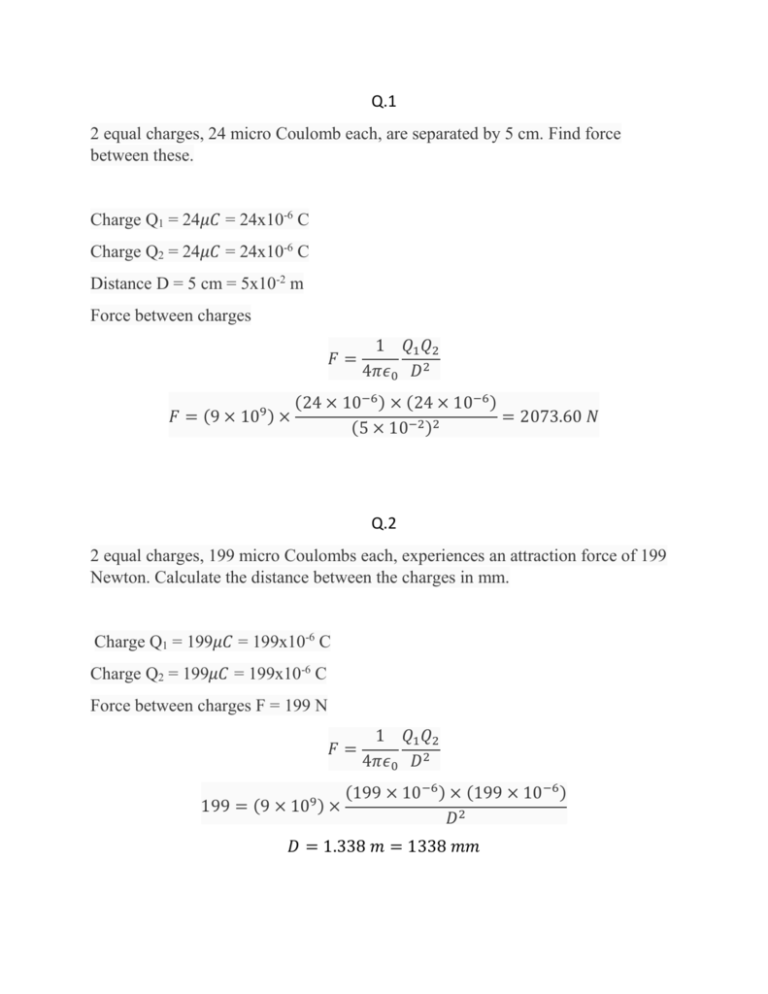
Q.1 2 equal charges, 24 micro Coulomb each, are separated by 5 cm. Find force between these. Charge Q1 = 24𝜇𝐶 = 24x10-6 C Charge Q2 = 24𝜇𝐶 = 24x10-6 C Distance D = 5 cm = 5x10-2 m Force between charges 𝐹= 𝐹 = (9 × 10 9) 1 𝑄1 𝑄2 4𝜋𝜖0 𝐷2 (24 × 10−6 ) × (24 × 10−6 ) × = 2073.60 𝑁 (5 × 10−2 )2 Q.2 2 equal charges, 199 micro Coulombs each, experiences an attraction force of 199 Newton. Calculate the distance between the charges in mm. Charge Q1 = 199𝜇𝐶 = 199x10-6 C Charge Q2 = 199𝜇𝐶 = 199x10-6 C Force between charges F = 199 N 𝐹= 199 = (9 × 10 9) 1 𝑄1 𝑄2 4𝜋𝜖0 𝐷2 (199 × 10−6 ) × (199 × 10−6 ) × 𝐷2 𝐷 = 1.338 𝑚 = 1338 𝑚𝑚 Q.3 Add 0.005 Coulombs, 2,555 micro Coulombs and 363,286 nano Coulombs and express the result in micro Coulombs. Charges 𝑄1 = 0.005𝐶 = 0.005 × 106 𝜇𝐶 = 5000 𝜇𝐶 𝑄2 = 2555𝜇𝐶 𝑄3 = 363286 𝑛𝐶 = 363286 × 10−3 𝜇𝐶 = 363.286𝜇𝐶 Total Charge 𝑄 = 5000 + 2555 + 363.286 = 7918.286 𝜇𝐶 Q.4 2 equal charges are separated by 37 mm and experiences a repulsion force of 9 Newton. Calculate the amount of each charge in micro Coulombs. Charge Q1 = Q2 = Q Distance D = 37mm = 37x10-3 m Force between charges F = 9N 𝐹= 9 = (9 × 10 1 𝑄1 𝑄2 4𝜋𝜖0 𝐷2 9) 𝑄2 × (37 × 10−3 )2 𝑄 = 1.17 × 10−6 𝐶 = 1.17𝐶 Q.5 What is the total charge of all protons in 3 gram of water (H2O)? Molecular weight of H2O= 2x1+16=18 Moles in 3 gms = 3/18 = 1/6 M Number of molecules = moles x Avogadro constant = 1.004 × 1023 1 6 × (6.022 × 1023 ) = Now 1 molecule of H2O has 2 Hydrogen atoms and 1 Oxygen atom. Hydrogen has 1 proton per atom and Oxygen has 8 protons per atom. Thus 1 molecule of H2O has total 10 (1x2+8) protons per molecule. So total number of protons in 3 gms water = (1.004 x 1023) x 10 =1.004 x 1024 Charge on one proton = 1.6x10-19C So total charge on protons = (1.004 x 1024) x (1.6 x 10-19) = 160640 C Q.6 How many excess electrons can be counted in a rubber rod of - 1 *0.00001 nano Coulombs of charges? Total charge = -1 x 0.00001 nC = -1 x 10-5 nC = (-1 x 10-5) x 10-9 C= -1 x 10-14 C Charge on one electron = - 1.6 x 10-19C Number of electrons = −1×10−14 −1.6×10−19 = 62500 Q.7 A metal ball has - 2 Coulomb of charge. If it receives 0.50*10^20 number of electrons, what will be the resultant charge of the ball? Original charge = - 2C Added electrons = 0.50 x 1020 Charge on one electron = - 1.6 x 10-19C So newly added charge = (0.50 x 1020) x (-1.6 x 10-19) = - 8C Net charge = -2 -8 = -10 C Q.8 How many electrons have to be added to 0 kg metal sphere such that another +1C charge located 2 mm above the sphere will be able to hold that in the air?[Assume g=10m/s^2]. Data insufficient as mass of sphere is given 0 Kg Assuming mass is 𝑚 Kg (known) and charge is Q C (unknown) Distance = 2 mm = 2 x 10-3 m Gravitational force 𝐹1 = 𝑚𝑔 = 𝑚 × 103 × 10 = 10000𝑚 𝑁 Attraction force 𝐹2 = 1 𝑄1 𝑄2 1×𝑄 9) (9 = × 10 × = 2.25 × 1015 𝑄 𝑁 (2 × 10−3 )2 4𝜋𝜖0 𝐷2 Under equilibrium 𝐹1 = 𝐹2 2.25 × 1015 𝑄 = 10000𝑚 𝑄 = 4.444 × 10−12 𝑚 𝐶 Number of electrons = 𝑄 1.6×10−19 = 1.7 × 107 𝑚