The Diabetes and Pre-eclampsia Intervention Trail
advertisement

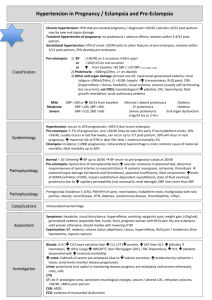
Pregnant women with type 1 diabetes mellitus are at increased risk of developing pre-eclampsia, which presents as hypertension or high blood pressure and proteinuria (protein present in urine). Somewhere between 13 and 30% of pregnant women with diabetes will develop high blood pressure during pregnancy, and this is a major cause of hospital admissions. Pre-eclampsia is an important cause of premature delivery and low birth weight in babies, and at present delivery of the baby is the only known cure. A reduction in the rate of pre-eclampsia would therefore have major benefits in terms of improving the health of both the mother and the baby. At present, the cause of pre-eclampsia is not fully understood. However, it has been suggested that it is, in part, due to a poor blood supply to the placenta. This may be due to the production of harmful chemicals called free radicals which damage the cells lining the blood vessels in the placenta. Recently it has been suggested that taking supplements of vitamin C and vitamin E during pregnancy may prevent the production of harmful free radicals and thus reduce the risk of pre-eclampsia. A study in 283 women without diabetes showed that taking supplements of vitamin C and vitamin E reduced the occurrence of preeclampsia from 17% to 8% (Chappell et al. Lancet 1999; 354: 810-816). However, there were no women with diabetes in this study and it is unclear whether a similar benefit will be found in the presence of diabetes. Study Aim: To determine whether supplementation with vitamin C and vitamin E from early pregnancy will reduce the risk of developing pre-eclampsia in pregnant women with type 1 diabetes. Study Design: Double-blind randomised multicentre placebo-controlled trial Intervention: Vitamin C 1000mg and Vitamin E 400IU or placebo tablets daily Patients: 756 women from 3 UK regions will be recruited to participate in this trial Eligibility: Women with type 1 diabetes preceding pregnancy, presenting between 8 and 22 weeks gestation Primary outcome: Incidence of pre-eclampsia Secondary outcomes: endothelial activation; birthweight centile Study Duration: Recruitment commenced in March 2003. The trial is now fully recruited. The last baby was delivered in December 2008. The trial report is expected early in 2010. DAPIT Protocol Version 1.2: Click here to view protocol. Funding Source: The Wellcome Trust ISRCTN27214045 DAPIT Centres: Northern Ireland: Belfast Royal Jubilee Maternity Service (Dr David McCance) Ulster Hospital, Dundonald (Dr Roy Harper) Craigavon Craigavon Area Hospital (Dr Kate Ritchie) North-West Altnagelvin Hospital (Dr Ken Moles) Antrim Antrim Area Hospital (Dr Adele Kennedy) Scotland: Edinburgh Edinburgh Royal Infirmary (Dr Alan Patrick) Western General Hospital, Edinburgh (Dr Corinne Love) St John’s Hospital, Livingston (Dr James Walker) Glasgow Princess Royal Maternity Hospital, Glasgow (Dr Ken Paterson) Queen Mother’s Maternity Hospital, Glasgow (Dr Alan Cameron) Southern General Hospital, Glasgow (Dr Andy Gallagher) Central Scotland Wishaw General Hospital (Dr Dina McLellan) Forth Valley Stirling Royal Infirmary (Dr Hilary MacPherson) Falkirk & District Royal Infirmary (Dr Hilary MacPherson) Dundee Ninewells Hospital, Dundee (Dr Graham Leese) Aberdeen Aberdeen Maternity Hospital (Dr Donald Pearson) Fife Forth Park Maternity Hospital (Dr Rennie Urquhart) NW England: Manchester St Mary’s Hospital for Women & Children (Dr Mike Maresh) Hope Hospital, Salford (Dr Bob Young) Liverpool Liverpool Women’s Hospital (Mr Steve Walkinshaw) Aintree University Hospital (Dr Ian Casson) Birmingham / West Midlands Birmingham Women’s Hospital (Mr Harry Gee) Good Hope Hospital (Dr John Milles) City Hospital (Dr Ansu Basu) Walsall Hospital (Mrs Balachandar) If you require further information regarding the trial, or wish to be put in contact with one of the DAPIT Research Midwives at any of the participating centres, please contact the DAPIT Clinical Coordinating Centre on 028 9063 2524. DAPIT Trial Coordinator: Dr Valerie Holmes, DAPIT, Institute of Clinical Sciences, Block B, Grosvenor Road, Belfast BT12 6BW Email: v.holmes@qub.ac.uk Telephone: 028 9063 2524 Principal Investigator: Dr David R McCance, Consultant Physician / Honorary Senior Lecturer, Regional Centre for Endocrinology and Diabetes, Royal Victoria Hospital, Belfast BT12 6BA Investigators page (available to study members only)