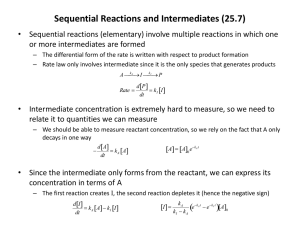
Rate Laws & Reaction Orders

Honors Chemistry
Name: ______________________________________ Date: _______________ Mods: ___________
Ch. 14 – Chemical Kinetics: Rate Laws & Reaction Orders
1. Rate Laws:
The ________________________ of one or more of the reactants may impact the reaction rate, or the rate may be ______________________ of the concentrations. The dependence of the reaction upon reactant concentration is expressed in a rate law equation:
For the balanced equation, A + B C
Rate = k [A] n [B] m (rate law expression) k is the rate constant, [A] and [B] are the molar concentrations of the reactants, and n and m are the exponents that reflect how the rate depends on the reactant concentrations.
The rate constants and the exponents n and m __________________ be determined merely be looking at the balanced chemical equation; they must be determined
__________________________.
The rate constants for a reaction depend on the temperature, not on the reactant concentrations. At a higher temperature, the entire rate of the reaction ___________________ while at lower temperatures, the rate decreases.
The exponents n and m give the _________________ of the reaction. Thus, reactant A is said to be of the n th order and reactant B to be of the m th order of the above reaction o exponents are usually positive whole numbers, but they may be fractions or may even be negative numbers.
The sum of n plus m is the overall reaction order .
2. Orders of Reaction:
Since t he exponent’s n and m vary with each reaction, the rate laws for different reactions take on different forms. The three examples below will illustrate this fact.
Zero Order: If a reactant has an order of zero that means the rate is ____________________ of the reactant’s concentration and that reactant does not _________________ in the rate law at all (because anything raised to the power of zero is equal to one). As seen in the example below, the order of CO is zero and hence [CO] does not appear in the rate law. This means that increasing or decreasing the concentration of a zero order reactant will have ________
__________________ on the rate of the reaction o Example: NO
2
(g) + CO (g) NO (g) + CO
2
(g) Rate = k [NO
2
] 2
First Order: When a reactant is first order, the reactant will appear in the rate law and have an exponent of 1 (this exponent of 1 is not typically written in the rate law). As seen in the example below, both reactants (F
2
and ClO
2
) have an order of 1. Increasing the concentration of F
2
or ClO
2
___________________ the rate and decreasing the concentration of F
2
or ClO
2
_________________ the rate. If the concentration of F
2
is doubled, for instance, the rate of the reaction will ____________. If the concentration of F
2
is halved, the rate will also be cut in half.
Thus, the rate of the reaction is _____________ proportional to any reactant that is first order. o Example: F
2
(g) + 2 ClO
2
(g) 2 FClO
2
(g) Rate = k [F
2
][ClO
2
]
Second Order: Reactants that are second order will appear in the rate law as the concentration of the reactant squared. As seen in the example below, [NO] appears in the rate law and is squared, hence NO has an order of 2. As a result, if the concentration of NO is doubled this will cause the rate to increase by a factor of _______. If the concentration of NO is tripled, the rate will increase by a factor of _______. o Example: 2 NO (g) + H
2
(g) N
2
(g) + H
2
O (g) Rate = k [NO] 2 [H
2
]
The most important point about rate laws is that the order of each reactant in the rate law determines how the rate changes as the concentration of each reactant changes.
Example Problems:
1) The reaction C
6
H
5
N
2
Cl (aq) + H
2
O (l) C
6
H
5
OH (aq) + N
2
(g) + HCl (aq) is first order in
C
6
H
5
N
2
Cl and zero order in H
2
O. What is the rate law expression?
2) For the reaction 2 NO (g) + Cl
2
(g) 2 NOCl (g) If the concentration of NO is tripled, the rate of the reaction increases by a factor of nine. If the concentration of Cl
2
is cut in half, the rate of the reaction is decreased to half the original rate. Find the order of reaction for each reactant and write the rate expression for the reaction.
3) What is the overall order of reaction for each of the following? a) Rate = k [NO
2
] 2 b) Rate = k c) Rate = k [H
2
][Br
2
] 1/2 d) Rate = k [NO] 2 [O
2
]
4) For the reaction H
3
PO
4
(aq) + 3
I –
(aq) + 2 H + (aq) H
3
PO
3
(aq) +
I
3
–
(aq) + H
2
O (l) the rate expression under certain conditions is given by Rate = k [H
3
PO
4
][ I –
][H + ] 2 . What method(s) could be used if you want to double the reaction rate?
3. Determining a Rate Law from Quantitative Data:
1) Use the following reaction as an example of how a rate law can be determined from experimental data:
Trial 1
Trial 2
Trial 3
2 NO (g) + O
2
(g) 2 NO
2
(g) ; Rate = k [NO] n [O
2
] m
Experimental Data from the Reaction of NO (g) with O
2
(g)
Initial Concentration [NO] Initial Concentration [O
2
] Initial Rate
0.01
0.02
0.02
0.02
0.025
0.105
0.01 0.04 0.05 a) Determining the order with respect to NO:
From trials 1 & 2 in the data above, the concentration of O
2
is held constant (at 0.02 M) while the concentration of NO is doubled (from 0.01M to 0.02 M), therefore, any change in the reaction rate will be due to the change in the NO concentration.
We can now look at the in [NO] and changes in the initial rate to evaluate their relationship:
Concentration [NO]: Initial Rate:
Trial 2 =
Trial 1
From this evaluation, the order of NO is _________. b) Determining the order with respect to O
2
:
From trials 1 & 3 in the data above, the concentration of NO is held constant (at 0.01 M) while the concentration of O
2
is doubled (from 0.02M to 0.04 M), therefore, any change in the reaction rate will be due to the change in the O
2
concentration.
We can now look at the in [O
2
] and changes in the initial rate to evaluate their relationship:
Concentration [O
2
]: Initial Rate:
Trial 3 =
Trial 1 c) Therefore, the rate law for the reaction 2NO (g) + O
2
(g) 2 NO
2
(g) is:
From this evaluation, the order of O
2
is _________. d) The overall reaction order is: ________. e) The value of k, the rate constant is:
2) Use the following reaction as an example of how a rate law can be determined from experimental data:
Trial 1
Trial 2
Trial 3
W + X Y + Z ; Rate = k [W] n [X] m
Experimental Data from the Reaction of W with X
Initial Concentration [W] Initial Concentration [X] Initial Rate
0.1 0.1 1.02 x 10 -4
0.1
0.3
0.2
0.1
1.00 x 10 -4
3.05 x 10 -4 a) Determining the order with respect to W:
From this evaluation, the order of W is _________. b) Determining the order with respect to X:
From this evaluation, the order of X is _________. c) Therefore, the rate law for the reaction 2NO (g) + O
2
(g) 2 NO
2
(g) is: d) The overall reaction order is: ________. e) The value of k, the rate constant is: f) In a 4 th trial, the concentration of [W] is 0.33 M and the concentration of [X] is 0.41 M.
Determine the rate of the reaction with these given concentrations.
Reaction Rate Laws & Orders WS
1) A reaction has the experimental rate law of Rate = k [A] 2 . a. What happens to the rate if the concentration of A is tripled? b. What happens to the rate if the concentration if A is reduced to one third the initial concentration?
2) The reaction 2 NO
2
(g) + O
3
(g) N
2
O
5
(s) + O
2
(g) is first order with respect to each reactant. a. Write the rate expression for the reaction b. How does doubling the concentration of NO
2
affect the reaction rate? c. How does tripling the concentration of O
3
affect the reaction rate?
3) For the reaction 2 A + B C + D, if the concentration of A is doubled, the reaction rate doubles. If the concentration of B is halved, there is no change in the reaction rate. Determine the order of reaction with respect to each reactant and the overall order of reaction. Write the rate law for the reaction.
4) For the reaction A + 2B AB
2
, the following data were obtained.
Trial 1
Trial 2
Trial 3
Initial [A]
0.66
0.66
0.22
Initial [B]
0.47
0.94
0.47
Initial Rate (M/hr)
0.370
1.480
0.123 a. Determine the order with respect to each reactant : b. Determine the overall order of reaction: c. Write the rate expression for the reaction: d. Find the value of the rate constant, k:
5) For the reaction A + B AB , the following data were obtained.
Trial 1
Trial 2
Trial 3
Initial [A]
0.480
0.240
0.480
Initial [B]
0.380
0.190
0.190
Initial Rate (M/hr)
0.346
0.087
0.350 a. Determine the order with respect to each reactant : b. Determine the overall order of reaction: c. Write the rate expression for the reaction: d. Find the value of the rate constant, k:
6) For the reaction 2A + B
2
2 AB , the following data were obtained.
Trial 1
Trial 2
Trial 3
Initial [A]
0.3
0.15
0.3
Initial [B]
0.43
0.43
1.72
Initial Rate (M/hr)
0.34
0.34
1.36 a. Determine the order with respect to each reactant : b. Determine the overall order of reaction: c. Write the rate expression for the reaction: d. Find the value of the rate constant, k:
7) Consider the reaction A + 2B C. The rate law for this reaction is second order in A and second order i n B. If the rate constant at 25ᵒC is 1.25x10
-2 s -1 , write the rate law and find the rate of reaction when the concentration of A is 0.27 M and the concentration of B is 0.32 M.
8) Consider the reaction A + 2B C + D. The rate law for this reaction is first order in A and first order in B. If the rate constant at 25 C is 1.94x10
2 s -1 , write the rate law and find the rate of reaction when the concentration of A is 0.68 M and the concentration of B is 0.14 M.
9) Consider the reaction 2A + B C + 2D. The rate law for this reaction is first order in A and first order in B. If the rate constant at 25 C is 3.01x10
2 s -1 , write the rate law and determine the rate of reaction when the concentration of A is 0.47 M and the concentration of B is 0.79 M.