ICH602S Practical 1 Detection of Cations 21 july 2015
advertisement

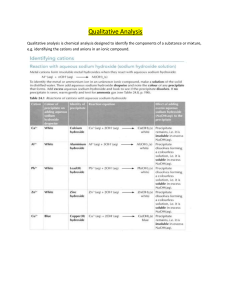
Inorganic Chemistry (ICH602S) Practical 1: Detection of Cations Date: 21 July 2015 1. Introduction Cations in an aqueous Solution can be Identified by using sodium hydroxide solution and aqueous ammonia solution. All hydroxides are insoluble except Group I metal Hydroxides and Calcium hydroxide (limewater–sparingly soluble). Therefore, when an aqueous metal (non group I) cation reacts with a hydroxide anion (OH--‐--‐ the hydroxide anion can come from sodium Hydroxide or aqueous ammonia), an insoluble metal hydroxide is formed, leading to the observation of a precipitate. The reaction can be represented as follows: Mn+(aq) + nOH- (aq) → M(OH)n (s) On addition of excess reagent, certain metal hydroxides can react with either more hydroxide anions or ammonia to form a soluble metal complex. Therefore, the precipitate dissolves. For example, Zn2+: Addition of sodium hydroxide / aqueous ammonia dropwise Zn2+ (aq) + 2OH‐ (aq) → Zn(OH)2 (s) Observation: white ppt forms In summary, the unknown cation(s) can be identified by noting a) The colour of the precipitate produced (if any); b) If the precipitate is soluble in excess of the reagent. 2. Practical steps a. Take between 1 – 2 cm test--tube height of the unknown solution containing the metal cation. b. Add sodium hydroxide solution or aqueous ammonia dropwise, with shaking. c. Observe the formation of any precipitate. d. If there is a precipitate, note the colour of the precipitate. e. Then add the sodium hydroxide solution to excess. f. Observe if the precipitate (if any) dissolves. If it does, note the colour of the solution formed.