Determining Empirical & Molecular Formulas
advertisement
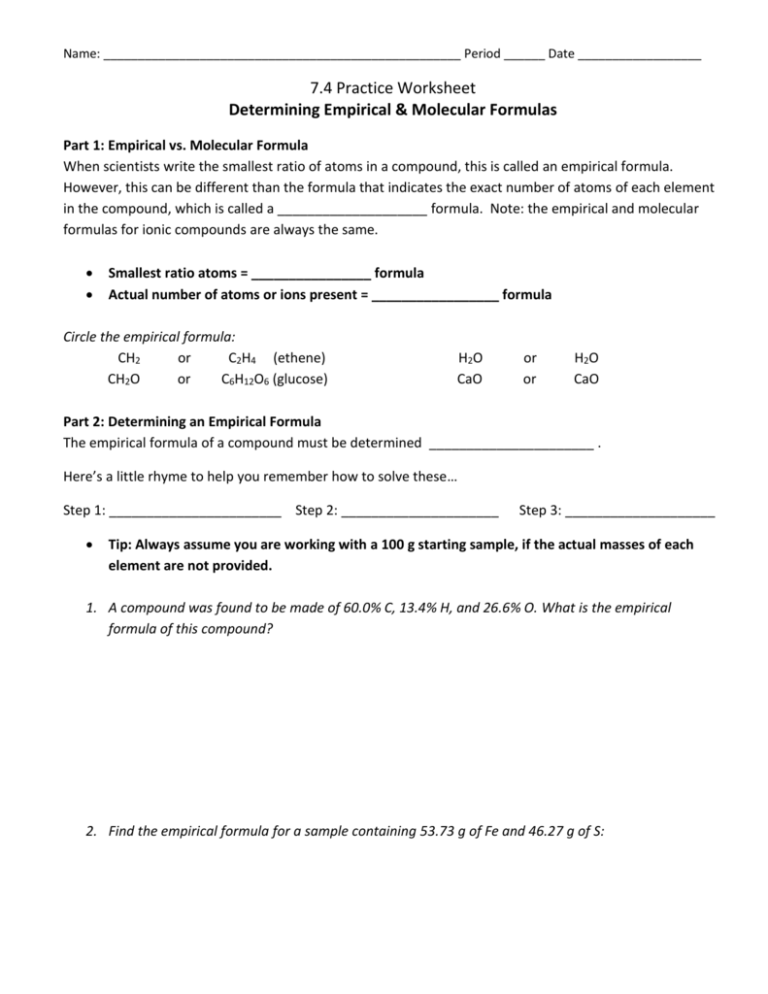
Name: ____________________________________________________ Period ______ Date __________________ 7.4 Practice Worksheet Determining Empirical & Molecular Formulas Part 1: Empirical vs. Molecular Formula When scientists write the smallest ratio of atoms in a compound, this is called an empirical formula. However, this can be different than the formula that indicates the exact number of atoms of each element in the compound, which is called a ____________________ formula. Note: the empirical and molecular formulas for ionic compounds are always the same. Smallest ratio atoms = ________________ formula Actual number of atoms or ions present = _________________ formula Circle the empirical formula: CH2 or C2H4 (ethene) CH2O or C6H12O6 (glucose) H2O CaO or or H2O CaO Part 2: Determining an Empirical Formula The empirical formula of a compound must be determined ______________________ . Here’s a little rhyme to help you remember how to solve these… Step 1: _______________________ Step 2: _____________________ Step 3: ____________________ Tip: Always assume you are working with a 100 g starting sample, if the actual masses of each element are not provided. 1. A compound was found to be made of 60.0% C, 13.4% H, and 26.6% O. What is the empirical formula of this compound? 2. Find the empirical formula for a sample containing 53.73 g of Fe and 46.27 g of S: 3. Find the empirical formula for a compound that contains 15.77% Al, 28.11 % S, and 56.12% O: Part 3: Determining a Molecular Formula (true formula) If you know the empirical formula of a compound and its molecular mass (true molar mass), it is possible to determine its molecular formula. (Of course, if the empirical formula is not provided, you will have to do that step first). 1. What is the molecular formula of the compound with empirical formula C3H8O and a molecular mass of 180.33 g/mol? 2. A compound is found to be 40.0% carbon, 6.70 % hydrogen, and 53.5% oxygen. Its molecular mass is 60.0 g/mol . What is its molecular formula? 3. A compound is 64.9% C, 13.5% H, and 21.6% O . Its molecular mass is 74.0 g/mol . What is its molecular formula?











