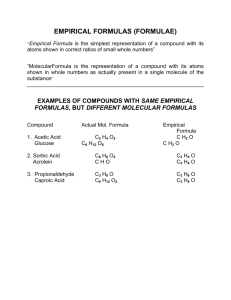
Empirical and Molecular Formulas Definitions: Empirical Formula
advertisement

Empirical and Molecular Formulas Definitions: Empirical Formula: Lowest whole number ratio of atoms in a molecule. Molecular Formula: the exact number of atoms of each element in the smallest unit of a substance. Problem 1 Problem Solving Techniques, Do at home before class: Your friend builds you many of the lego butterflies shown and leaves them on your end table for you to find. They are all identical and have identical numbers of pieces. Sadly your pet dog ruins them before you are able to count how many your friend made. a) If you count 4000 yellow legos, 2000 black legos, 1200 blue legos and 400 orange legos, what is the lowest whole number ratio of legos? Write it like you would a chemical formula YwBkxBuyOz. (This is like your empirical formula). b) If you talk to your friend and they tell you that originally there were 4 butterflies, how many legos were present in each butterfly? Write it like you would a chemical formula YwBkxBuyOz. (This is like your molecular formula) Problem 2: The compound sold under the name Prozac is 66.01% C, 5.87% H, 18.43% F, 4.53% N and 5.17% O. What is the empirical formula? Empirical and Molecular Formulas Problem 3: Conceptual Frame work: If we combust a hydrocarbon (CxHyOz), H2O and CO2 can be absorbed and measured. All H comes from the hydrocarbon, all C comes from the hydrocarbon. Oxygen comes from both the hydrocarbon and the O2. A folk medicine used in the Anhui province of China to treat acute dysentery is cha-tiao-qi, a preparation of the leaves of Acer ginnala. Reaction of one of the active ingredients in cha-tiao-qi with water yields gallic acid, a powerful antidysenteric agent. Gallic acid is known to contain carbon, hydrogen and oxygen. A chemist wanting to determine the molecular formula of gallic acid burned 1.000g of the compound in an elemental analyzer. The products of the combustion were 1.811 g of CO2 and 0.3172 g of H2O. a) Determine the empirical formula of the compound. i) All carbon came from the hydrocarbon and became CO2 therefore, using this, find the moles of C. ii) All hydrogen came from the hydrocarbon and became H2O therefore, using this, find the moles of H. iii) Oxygen possibly came from two places, the H2O and the CO2, therefore you can’t use the same method. Instead, use the fact that the hydrocarbon all C, H and O, and you know you have a 1 g sample. iv) Now that you have the moles of each species, solve as in problem 2. Empirical and Molecular Formulas b) The molar mass was found to be 170.12 g/mol. What is the molecular formula of gallic acid? c) What would the formula be if the mass was found to be 510 g/mol? Problem 4: Challenge Question (We won’t likely have time for this one) Answers to be posted online. Difficulty level: **** A compound produced as a bi-product in an industrial synthesis of polymers was found to contain carbon, hydrogen and iodine. A combustion analysis of 1.70g of the compound produced 1.32 g of CO2 and 0.631g of H2O. The mass percentage of iodine in the compound was determined by converting the iodine in a 0.850 g sample of the compound into 1.15g of lead(II) iodide. What is the empirical formula of the compound? Could the compound also contain oxygen? Explain.