Lab: Airbag Stoichiometry
advertisement

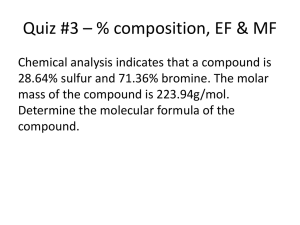
Name: _____________________________________________ Date:_________ period:_____ Unit 9 Stoichiometry Lab: Airbag Stoichiometry Essential Question: How much do I get when I mix these things together? Purpose: In this lab, you will design and construct a model “airbag” using stoichiometry. Safety Concerns Be sure to wear safety goggles at all times. Use caution when handling acetic acid (vinegar). Inflate your finished airbag only in a safe area determined by your teacher. Introduction The development of the airbag for automobiles required the combined efforts of both chemists and engineers. The basic idea is simple: in the event of a collision, a plastic bag rapidly inflates with a gas, preventing the occupant from hitting the dashboard or the steering column. There are still some unresolved issues regarding the use of air bags and the type of air bag that provides the most safety. With regard to the bag itself, it must: 1. Not inflate by accident. 2. Produce non-toxic materials. 3. Produce a gas that is cool. 4. Inflate very rapidly (20-60 milliseconds). 5. Be lightweight, easy to handle, and stable for long periods. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide (NaN3), producing nitrogen, an extremely unreactive gas. Write the reaction for the decomposition of Sodium Azide below: Sensors that detect an impact activate the air bag system. The sensor sends a current along a detonator, which causes a small explosion to start the decomposition of sodium azide. A few milliseconds later, about 60 liters of nitrogen gas inflate the bag. The sodium produced is very reactive and must be converted to a less hazardous material. The most common method involves the reaction with iron (III) oxide, otherwise known as “rust”: Write the reaction for the single replacement reaction of iron (III) oxide and sodium: Since sodium azide is extremely toxic (it is a carcinogen as well as a skin and eye irritant) you will use another gas-producing reaction to construct your airbag: the reaction between baking soda (sodium bicarbonate) and an acid to form carbon dioxide gas. In this case, we will be using acetic acid, the active ingredient in vinegar. The reaction is written as follows: **Sodium Bicarbonate + acetic acid sodium acetate + carbon dioxide + water NaHCO3 (s) + CH3CO2H (aq) NaCH3CO2 (aq) + CO2 (g) + H2O (l) Prelab Questions: 1. What are some of the considerations that must be made when designing an airbag? 2. What gas is used to fill car airbags? 3. About how much gas fills a typical airbag? 4. What is done with the reactive sodium after the reaction to make sure the products are safe? 5. What reaction will we be using in our lab instead of the decomposition of sodium azide? 6. What is the mole ratio of the sodium bicarbonate to acetic acid? (use the balanced equation given above). 7. Calculation: If 3 moles of NaHCO3 (s) are used, how many moles of CO2 (g) are formed? 8. How many liters of CO2 are formed (from number 7)? Procedure There isn’t one! Your object is to inflate the Ziploc bag provided with the carbon dioxide produced in the reaction. When inflated, the bag should be firm, but should not burst. You should optimize your system to inflate as rapidly as possible. We do not want acid flying around the lab because bags have burst under too much pressure. Therefore, you must first have your teacher check your calculations and procedure. The reaction will be performed at your station with each person at your station wearing goggles and using extreme caution. You should combine reactants over the sink. In order to get full credit for this lab, your airbag must inflate fully (without bursting) on the first or second attempt. If your bag does not inflate on the first attempt, you must revise your procedure before trying again. Your performance will be scored out of 5 points: 1st attempt: 7 points (full credit plus 2 bonus points) 2nd attempt: 5 points (full credit) 3rd attempt: 2 points Procedure: Write the procedure you and your lab group developed. Calculations: You need to use stoichiometry to determine how much of the reactants you need to inflate your bag. (Quantities of reactants must be approved before starting your experiment) Data Table: (quantities of reactants must be approved before starting your experiment) Trial # Grams of Moles of mL of Moles of Approx % Repeat NaHCO3 NaHCO3 vinegar- need vinegar bag filled trial for concentration 100% filled calculation Conclusion Questions 1. How many trials did it take for you to completely fill your bag? If it took more than one, what changes in your procedure did you make? (if you completed it in one trial, what improvements could you have made in your procedure?) 2. How many moles of baking soda did you use? Show your calculations. 3. With that many moles of baking soda- how many Liters of Carbon dioxide were produced? 4. What is the maximum amount of CO2 the baggies can hold without bursting (in liters and moles)? 5. Application question: If 60 Liters of Nitrogen gas fills a standard airbag, how many moles of Sodium azide must be used in an airbag? How many grams? 6. Application question: Using the information from problem 5, how many moles of sodium would be produced from that reaction? When combined with Fe2O3 in the next reaction, how many moles of Fe2O3 should be used in the airbag?