Gas Law Constant R Determination Lab Manual
advertisement

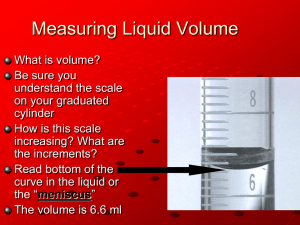
Unit 10: Determination of Gas Law Constant (R) Name: ________________________ Purpose: To experimentally calculate the value of the Ideal Gas Law constant, R. Introduction: We have been working with the Ideal Gas Law, PV = nRT. In this lab, we will measure the temperature, pressure, and volume of a known quantity of hydrogen gas. These values will then be used to calculate the value of the Ideal Gas Law constant, R. You will begin this experiment by collecting hydrogen gas formed by the reaction of magnesium metal with hydrochloric acid, represented by the following equation: Mg(s) + 2 HCl(aq) ===> MgCl2(aq) + H2(g) Once you have collected the hydrogen gas, you will measure the temperature, pressure and volume of the gas collected. Since the hydrogen gas is being collected over water, it will be saturated with water vapor. According to Dalton’s Law of Partial Pressures, the total pressure of the gas mixture is the sum of the partial pressure of H 2 and the partial pressure of the water vapor. You will use the chart below to determine the partial pressure of the water vapor and subtract from the total pressure to determine the pressure of H 2. The volume of the gas can be read from the collection tube. Pre-lab Questions 1. How will we determine the values of a. P? b. V? c. n? d. T? 2. Why is it important to tap the side of the graduated cylinder before reading the volume of gas collected? Materials and Equipment 4.0-cm ribbon of magnesium length of copper wire (reusable) 6M HCl (aq) 50-mL graduated cylinder stopper with hole(s) burette stand large beaker thermometer small funnel small graduated cylinder barometer electronic balance sandpaper Experimental Procedure Magnesium Ribbon 1. Obtain a ribbon of magnesium (Mg), a piece of sandpaper, and a length of copper wire. Use the pre-lab calculations to help you determine how much Mg to obtain. 2. Carefully sand the outside of the Mg ribbon to remove any oxide coating. Do not sand on the bench top! Place the Mg ribbon on a paper towel while sanding. Weigh the cleaned Mg ribbon and record this mass on your report form. 3. Wrap the Mg around the end of the copper wire. Do this in a tight ball with only a small gap between layers. Then wrap the copper wire to form a cage around the Mg ball. The cage must be tight enough to keep the Mg inside, but loose enough to allow water to easily flow around the wire. Roughly 3-cm of copper wire should be left over as a “handle” (see Figure 1). Figure 1 Before Inverting Figure 2 After Inverting Graduated Cylinder Set Up and Reaction 4. Obtain a graduated cylinder and stopper (with holes) from the instructor. 5. Fill your largest beaker ¾ full with tap water. 6. Add ~25mL of 6M HCl (aq) to the graduated cylinder using a small funnel. Then carefully add tap water to the graduated cylinder until it is filled to the brim. Pour the water down the side of the cylinder slowly being careful not to disturb the HCl. Make sure that the graduated cylinder is completely filled with water and no air bubbles remain. 7. Hang the Mg ball inside the open end of the graduated cylinder, ~2 cm down from the top. Then insert the stopper into the end. While holding the stopper in place, quickly invert the entire tube into your beaker filled with water. Clamp the graduated cylinder in the water in the upside down position (see Figure 2). 8. The reaction will occur as soon as the acid diffuses down the tube and reaches the Mg ribbon. As hydrogen gas is generated it will fill the graduated cylinder by forcing the water out of the tube and into the beaker via water displacement (see Figure 2). Allow the reaction to proceed until no Mg is left and no further gas is formed. This should take 3-5 minutes. Pressure Equalization 9. To ensure that the pressure of hydrogen (and water vapor) in the graduated cylinder is equal to atmospheric pressure, the level of the water inside the tube must be the same as the level of water outside the tube. To achieve this, raise or lower the tube until the internal and external water levels are equal. Record the volume of gas in the graduated cylinder (in mL). Measurements 10. After equalizing the water levels, record the following measurements: a. The temperature of the hydrogen gas collected, in °C. It is acceptable to assume that the temperature of the hydrogen gas is the same as the temperature of the water bath. b. The atmospheric pressure (use the lab barometer), in mm Hg c. The vapor pressure of water at the above temperature (obtain from Table on page 2), in mm Hg Data and Observations: a. Mass of Mg (g): ________________ b. Total Pressure (mm Hg): ________________ c. Vapor Pressure of water vapor (in mm Hg): ______________ d. Temperature (oC):________________ e. Volume (mL): ________________ Calculations: 1. Moles of H2 gas: Using the mass of the Mg ribbon and the balanced equation for the reaction, perform stoichiometric calculations to determine the moles of H2 gas produced. n = _________ 2. Pressure of H2 gas: As discussed in the introduction, the H2 gas is mixed with water vapor. To determine the pressure of H2 we will use the following equation: Patm = Phydrogen + Pwater You can find Pwater from the chart given in the introduction. Please give final answer in atmospheres!! P = __________ 3. Volume of H2 gas: Convert your measured volume from mL to L. V = _________ 4. Temperature of H2 gas: Convert your measured temperature from oC to K. T = _________ 5. Determine the experimental value of R: Using the values of n, P, V, and T, calculate the value of R. R = _________ 6. Percent error: Using 0.0821 Latm/molK to be the theoretical value of R, calculate your percent error. 𝑎𝑐𝑡𝑢𝑎𝑙 𝑣𝑎𝑙𝑢𝑒 𝑝𝑒𝑟𝑐𝑒𝑛𝑡 𝑒𝑟𝑟𝑜𝑟 = 𝑡ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 𝑣𝑎𝑙𝑢𝑒 × 100 %error = _________