Chemistry Midterm Review Packet How to study: Answer all the
advertisement

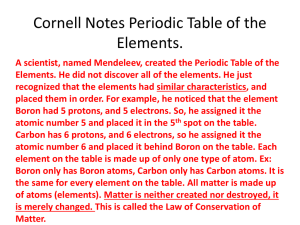
Chemistry Midterm Review Packet How to study: 1. 2. 3. 4. 5. 6. Answer all the objectives below. Define all vocab words. Answer course questions. Review old exams, quizzes, review packets, and book notes. Review lab notebook. Complete section and chapter reviews in the textbook. Objectives: 1. Define chemistry. 2. Describe the purpose of the scientific method. 3. List the steps of the scientific method. 4. Distinguish between qualitative and quantitative observations. 5. Contrast hypotheses, theories, laws, and models. 6. Distinguish between a quantity, a unit, a derived unit, and a measurement standard. 7. Name and use SI units for length, mass, time, volume, and density. 8. Describe how and why SI prefixes are used. 9. Use dimensional analysis to convert between units of measurement. 10. Distinguish between accuracy and precision. 11. Explain the purpose of significant figures in reporting measurements. 12. Determine the number of significant figures in measurements. 13. Convert measurements to and from scientific notation. 14. Distinguish between the physical properties and chemical properties of matter. 15. Classify changes of matter as physical or chemical. 16. Explain the gas, liquid, and solid states in terms of particles. 17. Explain how the law of conservation of energy applies to changes of matter. 18. Distinguish between a mixture and a pure substance. 19. Describe the arrangement of the periodic table. 20. List the characteristics that distinguish metals, nonmetals, and metalloids. 21. Summarize the five essential points of Dalton’s atomic theory. 22. Summarize the experiments of Thomson and Rutherford and their major conclusions. 23. List the properties of protons, neutrons, and electrons. 24. Explain what isotopes are. 25. Define atomic number and mass number. 26. Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. 27. Define mole, Avogadro’s number, and molar mass, and state how all three are related. 28. Solve problems involving mass in grams, amount in moles, and number of atoms of an element. 29. Explain the mathematical relationship between speed, wavelength, and frequency for electromagnetic radiation. 30. Discuss the dual wave-particle nature of light. 31. Discuss the significance of the photoelectric effect and the line-emission spectrum of hydrogen to the development of the atomic model. 32. Describe the Bohr model of the hydrogen atom. 33. List the four quantum numbers and their significance. 34. List the total number of electrons needed to fully occupy each main energy level. 35. Describe the electron configurations for atoms of any element using orbital notation, electronconfiguration notation, and noble-gas notation. 36. Explain the roles of Mendeleev and Moseley in the development of the periodic table. 37. Describe the modern periodic table. 38. Explain how periodic law can be used to predict the physical and chemical properties of elements. 39. Describe the relationship between electrons and period length in the periodic table. 40. Locate, name, and explain the four blocks of the periodic table. 41. Discuss the relationship between electrons and groups in the periodic table. 42. Describe the location on the periodic table and the properties of alkali metals, alkaline earth metals, halogens, and noble gases. 43. Compare periodic trends of atomic radii, ionization energy, and electronegativity, and state the reasons for these variations. 44. Define valence electrons and state how many are present in atoms of each main-group element. 45. Define chemical bond. 46. Explain why most atoms form chemical bonds. 47. Classify bonding type according to electronegativity differences. 48. Explain the relationships between potential energy, distance between approaching atoms, bond length, and bond energy. 49. State the octet rule. 50. List the six basic steps used in writing Lewis structures. 51. Explain how Lewis structures are used to show different types of bonds. 52. Compare and contrast the properties of single bonds and multiple bonds. 53. Describe the significance of resonance structures. 54. Discuss the arrangement of ions in crystals. 55. Compare and contrast the formula and properties of ionic and molecular compounds. 56. Describe the electron-sea model of metallic bonding and use it to explain why metals are good conductors of electricity. 57. Explain why metal surfaces are shiny. 58. Explain why metals are malleable and ductile but ionic-crystalline compounds are not. 59. Rank the strength of intermolecular forces (dipole-dipole forces, hydrogen bonding, induced dipoles, London dispersion forces) and describe their effects on properties such as boiling and melting points. Vocabulary: chemistry, chemical, scientific method, observing, quantitative, qualitative, system, hypothesis, control, variable, model, theory, law, quantity, SI, mass, weight, derived unit, volume, density, conversion factor, dimensional analysis, accuracy, precision, percentage error, significant figures, scientific notation, directly proportional, inversely proportional, matter, mass, volume, atom, element, compound, extensive property, intensive property, physical property, physical change, change of state, solid, liquid, gas, plasma, chemical property, chemical change, reactant, product, mixture, homogeneous, solution, heterogeneous, pure substance, family, period, metal, nonmetal, metalloid, atom, subatomic particle, proton, neutron, electron, nucleus, atomic number, isotope, mass number, nuclide, atomic mass unit, average atomic mass, mole, Avogadro’s number, molar mass, electromagnetic radiation, electromagnetic spectrum, wavelength, frequency, photoelectric effect, quantum, photon, ground state, excited state, line-emission spectrum, continuous spectrum, emission, absorption, quantum theory, orbital, quantum numbers, principal quantum number, angular momentum quantum number, magnetic quantum number, spin quantum number, electron configuration, Aufbau principle, Pauli exclusion principle, Hund’s rule, highest-occupied energy level, noble gas, noble gas configuration, periodic, periodic law, periodic table, lanthanides, actinides, periods, metal, alkali metals, alkaline-earth metals, transition elements (metals), main-group elements, nonmetal, metalloid, halogens, atomic radius, ion, ionization, ionization energy, second ionization energy, electron affinity, cation, anion, valence electrons, electronegativity, ionic bonding, covalent bonding, nonpolar-covalent bond, polar, polar-covalent bond, molecule, molecular compound, molecular formula, bond length, bond energy, octet rule, electron-dot notation, unshared pair, Lewis structures, structural formula, single bond, double bond, triple bond, multiple bonds, resonance, ionic compound, formula unit, crystal lattice, lattice energy, delocalized, sea of electrons (electron-sea), metallic bonding, malleability, ductility, enthalpy of vaporization, intermolecular forces, dipole, dipole-dipole forces, hydrogen bonding, London dispersion forces